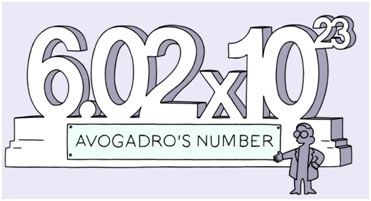Mole – A chemist’s secret unit
Definition:
A mole is specified as the quantity(mass) of a compound that contains 6.02 ×l023 number of particles (atoms, particles, or formula units).
It establishes a link between the mass of a compound and the number of particles of molar computations.
Representation: It is abbreviated as ‘mol’.
Explanation
We know that atom is an exceptionally little particle. The mass of an individual atom is an extremely small quantity. It is not possible to weigh individual atoms or even a small number of atoms directly. That is why we use the atomic mass unit(AMU) to express the atomic masses. For the sake of benefit, the atomic mass might be given up any unit of measurement i.e. grams, kg, pounds, and so on.
When the compound at our disposal is an element then the atomic mass of that component expressed in grams is called one gram atom. It is likewise called one-gram mole or just a mole of that element.
Number of gram atoms= Mass of an element in grams/
or moles of a componentMolar mass of a component
For example
1-gram atom of hydrogen = 1.008 g.
1-gram atom of carbon = 12.000 g.
and 1-gram atom of uranium = 238.0 g
It suggests that one gram atom of different components have different masses in them. One mole of carbon is 12 g, while 1 mole of magnesium is 24g. It likewise shows that an individual atom of magnesium is two times as heavy as an atom of carbon. The molecular mass of a compound expressed in grams is called gram molecule or gram mole or simply the mole of a substance.
Variety of gram particles or moles of a molecular substance = Mass of molecular substance in grams/ Molar mass of the substance.
For instance.
1gram molecule of water = 18.0 g.
1gram particle of H2SO4 = 98.0 g.
and 1gram molecule of sucrose = 342.0 g
It indicates that one gram molecule of different molecular compounds has different masses. The formula unit mass of an ionic substance expressed in grams is called the gram formula of the compound. Given that ionic compounds do not exist in molecular type therefore the sum of atomic masses of individual ions gives the formula mass. The gram formula is likewise described as gram mole or simply a mole.
Number of gram formulas or moles of a substance = Mass of the ionic substance in grams/ Solution mass of the ionic substance
1gram formula of NaCl = 58.50 g.
1gram formula of Na2CO3 = 106 g.
1gram formula of AgNO3 = 170g.
It might also be discussed here that the ionic mass of an ionic type expressed in grams is called one gram ion or one mole of ions.
For instance:
1 g ion of OH- = 17g.
1 g ion of SO42-= 96g.
1 g ion of CO3 2- =60g.
So, the atomic mass, molecular mass, formula mass, or ionic mass of the compound expressed in gram is called the molar mass of the substance.
Therefore, the quantitative meaning of mole is the atomic mass, molecular mass, or formula mass of a compound expressed in grams is called a mole.
Avogadro’s Number
Definition:
Avogadro’s number is the number of atoms, molecules, and ions in one gram atom of an element, one gram molecule of a compound, and one gram ion of a substance, respectively.
Representation: It is represented by the symbol ‘NA ‘.
Description
It is interesting to know that different masses of elements have the same number of atoms. Hence, the 6.02 ×l023number of atoms, molecules, or A formula units is called Avogadro’s number that is equivalent to one ‘mole’ of the respective compound. In basic words, 6.02 ×l023particles amount to one mole as twelve eggs are equal to one dozen.
To understand the relationship between Avogadro’s number and the mole of a substance let us consider a few examples.
- i. 6.02 ×l023atoms of carbon are equivalent to one mole of carbon.
- ii. 6.02 ×l023molecules of water are equivalent to one mole of water.
- iii. 6.02 ×l023formula units of NaCl are equivalent to one mole of sodium chloride.
Thus, 6.02 ×l023atoms of elements or 6.02 ×l023particles of molecular substance or 6.02 ×l023units of ionic substances are equivalent to 1 mole.
For further explanation about the number of atoms in molecular substances or the number of ions in ionic substances let us discuss two examples:
- One particle of water is comprised of 2 atoms of hydrogen and 1 atom of oxygen, hence 2×6.02 ×l023atoms of hydrogen and 6.02 ×l023atoms of oxygen make up one mole of water.
- One formula unit of sodium chloride includes one sodium ion and one chloride ion. So, there are 6.02 ×l023variety of Na+ ions and 6.02 ×l023CI– ions in one mole of sodium chloride. Therefore, the overall number of ions in 1 mole of NaCl is 12.04× l023 or 1.204× 1024.
Molar Volume
One mole of any gas at standard temperature and pressure (STP) occupies a volume of 22.414 dm3. This volume of 22.414 dm3 is called molar volume and it holds true only when the gas is ideal.
Ideal gas: An ideal gas is defined as one in which all collisions in between atoms or molecules are perfectly elastic and in which there are no intermolecular attractive forces. One can say it as a collection of completely hard spheres which collide but which otherwise do not interact with each other.
With the help of this information, we can convert the mass of a gas at STP into its volume and vice versa.
For this reason, we can state that.
- 2.016 g of H2 = 1 mole of H2 = 6.02 x 1023 molecules of H2 = 22.414 dm3 of H2 at S.T.P.
- 16g of CH4 = 1 mole of CH4 = 6.02 x 1023 particles of CH4= 22.414 dm3 of CH4 at S.T.P.
It is very intriguing to understand from the above information that 22.414 dm3 of each gas has a different mass however the very same number of particles. The reason is that the masses and the sizes of the molecules don’t affect the volumes. Generally, it is understood that in the gaseous state the distance between molecules is 300 times greater than their diameters.
Multiple-Choice Questions (MCQs) with Answers:
- What is a mole in chemistry?
- A. A small particle
- B. A unit for measuring mass
- C. 6.02 × 10^23 particles of a substance
- D. A type of chemical reaction
- Answer: C
- How is a mole represented in chemical equations?
- A. mol
- B. M
- C. mo
- D. mL
- Answer: A
- Which statement is true about one gram atom of hydrogen?
- A. It weighs 1.008 kg
- B. It is also called a mole of hydrogen
- C. It contains 6.02 × 10^23 hydrogen atoms
- D. It is heavier than 1 gram atom of carbon
- Answer: C
- What does Avogadro’s number represent?
- A. The mass of an element
- B. The number of particles in one mole of a substance
- C. The temperature at which gases react
- D. The volume occupied by one mole of gas
- Answer: B
- How is Avogadro’s number denoted?
- A. A
- B. N
- C. AN
- D. NA
- Answer: D
- What is the relationship between Avogadro’s number and a mole?
- A. They are unrelated concepts
- B. Avogadro’s number is equal to one mole
- C. Avogadro’s number is double a mole
- D. Avogadro’s number is a fraction of a mole
- Answer: B
- What is the molar volume of one mole of gas at standard temperature and pressure (STP)?
- A. 24.414 dm3
- B. 22.414 dm3
- C. 20.414 dm3
- D. 26.414 dm3
- Answer: B
- Under what conditions is the molar volume of a gas true?
- A. High pressure and high temperature
- B. Low pressure and low temperature
- C. Standard temperature and pressure (STP)
- D. Any conditions
- Answer: C
- Which of the following is considered an ideal gas?
- A. A gas with strong intermolecular forces
- B. A gas with perfectly elastic collisions
- C. A gas with high pressure
- D. A gas with low temperature
- Answer: B
- What does one gram formula of NaCl represent?
- A. 58.50 g of NaCl
- B. 1 mole of NaCl
- C. 6.02 × 10^23 ions of NaCl
- D. 1.204 × 10^24 ions of NaCl
- Answer: C
- What is the molar mass of a substance?
- A. The mass of one mole of the substance
- B. The volume of one mole of the substance
- C. The density of one mole of the substance
- D. The temperature of one mole of the substance
- Answer: A
- Which statement about the relationship between the masses and sizes of gas molecules is true?
- A. Masses and sizes affect volumes in gases.
- B. Masses affect volumes, but sizes do not.
- C. Sizes affect volumes, but masses do not.
- D. Neither masses nor sizes affect volumes.
- Answer: D
- What does one gram atom of uranium weigh?
- A. 238.0 kg
- B. 238.0 g
- C. 23.8 g
- D. 2.38 g
- Answer: B
- Which of the following is NOT a type of molar computation?
- A. Gram molecule
- B. Gram ion
- C. Mole molecule
- D. Gram formula
- Answer: C
- In one mole of water, how many atoms of hydrogen are there?
- A. 6.02 × 10^23
- B. 1.204 × 10^24
- C. 3.01 × 10^23
- D. 2.0 × 10^23
- Answer: A
Frequently Asked Questions (FAQs):
- What is a mole in chemistry?
- A mole is a unit that specifies the quantity (mass) of a compound containing 6.02 × 10^23 particles, be it atoms, molecules, or formula units.
- How is a mole represented in chemical equations?
- It is abbreviated as ‘mol’ in chemical equations.
- What is the relationship between a gram atom and a mole?
- One gram atom of an element is the atomic mass of that element expressed in grams, and it is equivalent to one mole of that element.
- How is the molar mass of a molecular substance calculated?
- The molar mass is calculated by dividing the mass of the molecular substance in grams by its molar mass.
- What is Avogadro’s number, and what does it represent?
- Avogadro’s number is 6.02 × 10^23, representing the number of atoms, molecules, or ions in one gram atom, gram molecule, or gram ion, respectively.
- How does Avogadro’s number relate to the mole of a substance?
- Avogadro’s number is equivalent to one mole of a substance, meaning 6.02 × 10^23 particles are equal to one mole.
- What is the molar volume of a gas at standard temperature and pressure (STP)?
- One mole of any gas at STP occupies a volume of 22.414 dm^3, and this volume is known as the molar volume.
- Under what conditions is the molar volume of a gas true?
- The molar volume is true only when the gas is ideal and under standard temperature and pressure conditions.
- What is an ideal gas?
- An ideal gas is one in which all collisions between atoms or molecules are perfectly elastic, and there are no intermolecular attractive forces.
- How can the mass of a gas at STP be converted into its volume and vice versa?
- The conversion can be done using the molar volume, where 1 mole of any gas at STP is equivalent to 22.414 dm^3.
- Does the molar volume vary for different gases at STP?
- No, the molar volume is the same for all gases at STP, regardless of the different masses of the gases.
- What factors do not affect the volume of gases in the gaseous state?
- The masses and sizes of gas molecules do not affect the volumes of gases in the gaseous state.
- What is the formula for calculating the number of moles of an ionic substance?
- The number of moles is calculated by dividing the mass of the ionic substance in grams by its molar mass.
- How is the molar mass of an ionic substance expressed in grams?
- The molar mass of an ionic substance is expressed as one gram ion or one mole of ions.
- Why is the molar volume of gases under ideal conditions?
- The molar volume is ideal because it assumes perfectly elastic collisions and the absence of intermolecular attractive forces between gas molecules.
Problems/Solutions Based On Mole Concept
Problem 1:
Calculating Moles from Mass
Question: Determine the number of moles in 45 grams of sodium chloride (NaCl).
Solution: To find the moles, use the formula: moles = mass / molar mass.
- Molar mass of NaCl = 23 g/mol + 35.5 g/mol = 58.5 g/mol.
- Moles of NaCl = 45 g / 58.5 g/mol.
Answer: The number of moles in 45 grams of NaCl is approximately 0.77 moles.
Problem 2: Converting Moles to Particles
Question: How many atoms are there in 2 moles of oxygen (O2)?
Solution: Use Avogadro’s number (6.02 × 10^23) to convert moles to particles.
- Moles of O2 = 2.
- Number of atoms = 2 moles × 6.02 × 10^23 atoms/mole.
Answer: There are 1.204 × 10^24 atoms in 2 moles of oxygen.
Problem 3: Finding Mass from Moles
Question: Calculate the mass of 3 moles of sulfur dioxide (SO2).
Solution: Use the formula: mass = moles × molar mass.
- Molar mass of SO2 = 32 g/mol + (2 × 16 g/mol) = 64 g/mol.
- Mass of SO2 = 3 moles × 64 g/mol.
Answer: The mass of 3 moles of SO2 is 192 grams.
Problem 4: Stoichiometry Using Moles
Question: In the reaction 2H2 + O2 → 2H2O, if 4 moles of hydrogen (H2) react, how many moles of water (H2O) are produced?
Solution: Use the mole ratio from the balanced equation.
- Moles of H2O = 4 moles of H2 × (2 moles of H2O / 2 moles of H2).
Answer: When 4 moles of H2 react, 4 moles of H2O are produced.
Problem 5: Gas Volume Calculations
Question: What is the volume, in liters, occupied by 2 moles of nitrogen gas (N2) at STP?
Solution: Apply the molar volume of gases at STP (22.414 liters/mol).
- Volume = 2 moles × 22.414 liters/mole.
Answer: The volume occupied by 2 moles of N2 at STP is 44.828 liters.
Problem 6: Limiting Reactant Calculation
Question: In the reaction 3A + 2B → C, if 4 moles of A react with 3 moles of B, determine the limiting reactant.
Solution: Compare the mole ratio from the balanced equation to the actual ratio.
- Moles of A used = 4 moles.
- Moles of B used = 3 moles.
Answer: Since the mole ratio is 3:2, B is the limiting reactant.
Problem 7: Empirical Formula Determination
Question: Given a compound with 12 grams of carbon (C) and 4 grams of hydrogen (H), find the empirical formula.
Solution: Determine the moles of each element and find the simplest whole-number ratio.
- Moles of C = 12 g / 12 g per mole.
- Moles of H = 4 g / 1 g per mole.
Answer: The empirical formula is CH₄.
Problem 8: Reaction Stoichiometry
Question: In the reaction 4NH₃ + 5O₂ → 6H₂O + 4NO, calculate the moles of oxygen (O₂) required to completely react with 6 moles of ammonia (NH₃).
Solution: Use the mole ratio from the balanced equation.
- Moles of O₂ required = 6 moles of NH₃ × (5 moles of O₂ / 4 moles of NH₃).
Answer: 12.5 moles of O₂ are required to react with 6 moles of NH₃.
Summary
The tutorial introduces essential concepts in chemistry related to the mole, Avogadro’s number, and molar volume.
- Mole – A Chemist’s Secret Unit:
- Definition: A mole is the quantity (mass) of a compound containing 6.02 × 10^23 particles, linking the mass and number of particles.
- Representation: Abbreviated as ‘mol.’
- Explanation: It provides a way to express atomic masses, where one gram atom of an element is equal to its atomic mass in grams.
- Avogadro’s Number:
- Definition: Avogadro’s number (‘NA’) is the quantity of atoms, molecules, or ions in one gram of a substance, equal to 6.02 × 10^23.
- Description: It highlights that different masses of elements share the same number of atoms. Examples illustrate the equivalence of Avogadro’s number to one mole of a substance.
- Molar Volume:
- Definition: One mole of any gas at standard temperature and pressure (STP) occupies a volume of 22.414 dm^3, termed molar volume, valid for ideal gases.
- Ideal Gas: Defined by perfectly elastic collisions and the absence of intermolecular attractive forces.
- Conversion: Mass-to-volume and vice versa conversions are facilitated using molar volume. For example, 1 mole of H2 at STP equals 22.414 dm^3.
The tutorial emphasizes the quantitative significance of the mole in expressing atomic, molecular, formula, or ionic masses. Avogadro’s number establishes a connection between the mole and the quantity of particles, while molar volume provides a standard volume for gases at STP. Understanding these concepts is fundamental in various chemical calculations and analyses.