What is Ideal Gas?
An ideal gas is a theoretical gas made up of a set of randomly moving point particles that interact only through elastic collisions. The perfect gas principle works because it follows the ideal gas law, a simplified equation of state, and is open to analysis under statistical mechanics.
Ideal Gas Laws
The laws which deal with ideal gases are naturally called ideal gas laws and the laws are identified by the observational work of Boyle in the seventeenth century and Charles in the eighteenth century.
Boyle’s Law– states that for a given mass of gas held at a constant temperature, the gas pressure is inversely proportional to the gas volume.
Charles Law– states that for a given fixed mass of gas held at a constant pressure the gas volume is directly proportional to the gas temperature.
Ideal Gas Equation
The Ideal gas equation is the formula for the state of a theoretical ideal gas. It is a great approximation to the behaviour of lots of gases under numerous conditions, although it has several several limitations. The ideal gas equation can be written as
PV = n RT
Formulation of Ideal Gas Equation
While describing Boyle’s and Charles’s laws, some of the variables are held continuous throughout the changes produced in the gases. According to Boyle’s law.

According to Charles’s law.
V ∝ T (when n and P are held constant).
It is a well-known fact that volume of the provided gas at constant temperature level and pressure is directly proportional to the number of moles (Avogadro’s law).
V ∝ n (when P and T are held constant).
If we think for a moment that none of the variables are to be kept constant then all the above 3 relationships can be joined together.

The consistent suggested is R which is called the general gas constant.

The equation is called an ideal gas equation. It is likewise referred to as the general gas equation. This formula shows that if we have any quantity of an ideal gas then the product of its pressure and volume is equal to the product of number of moles, general gas constant, and absolute temperature. This equation is decreased to Boyle’s law, Charles’s law and Avogadro’s law, when appropriate variables are held constant.
PV = n RT, when T and n are held constant, PV = k (Boyle’s law).

Ideal Gas Constant R
The values and units of R can be determined by Avogadro’s principle really easily. Its value depends on the systems chosen for pressure, volume and temperature level. The volume of one mole of an ideal gas at STP (one atmospheric pressure and 273.16 K) is 22.414 dm3. Putting these values in the general gas equation will provide the value of R.
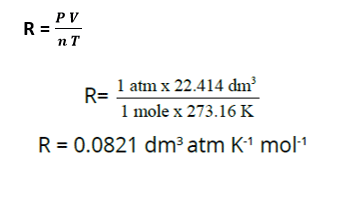
When the pressure is in atmospheres, volume in dm3, then the value of R, used needs to be 0.0821 dm3 atm K-1 mol-1.
The physical significance of this value is that, if we have one mole of an ideal gas at 273.16 K and one atmospheric pressure and its temperature is increased by 1 K, then it will take in 0.0821 dm3 -atm of energy, dm3 -atm is the system of energy in this circumstance. Thus, the value of R is a universal parameter for all the gases. It informs us that Avogadro’s number of molecules of all the perfect gases have the same demand for energy.
FAQs:
- What is an ideal gas?
- An ideal gas is a theoretical gas composed of point particles that move randomly and interact solely through elastic collisions. It follows the ideal gas law and is analyzed under statistical mechanics.
- What are the ideal gas laws?
- The ideal gas laws include Boyle’s Law and Charles’s Law. Boyle’s Law states that at constant temperature, gas pressure is inversely proportional to volume, while Charles’s Law states that at constant pressure, gas volume is directly proportional to temperature.
- What is the Ideal Gas Equation?
- The Ideal Gas Equation, often written as PV = nRT, describes the state of a theoretical ideal gas. It relates pressure (P), volume (V), temperature (T), and the number of moles (n) of a gas, with the universal gas constant (R) acting as a proportionality constant.
- How is the Ideal Gas Equation formulated?
- The Ideal Gas Equation is derived by combining Boyle’s Law, Charles’s Law, and Avogadro’s Law. It represents the relationship between pressure, volume, temperature, and number of moles for an ideal gas under various conditions.
- What is the significance of the Ideal Gas Constant (R)?
- The Ideal Gas Constant, denoted by R, is a universal parameter representing the behavior of ideal gases. Its value depends on the units chosen for pressure, volume, and temperature. It helps in calculating the energy required or released when the temperature of a gas changes.
- How is the value of the Ideal Gas Constant determined?
- The value of the Ideal Gas Constant can be determined by considering Avogadro’s principle and known conditions, such as one mole of gas at standard temperature and pressure (STP). The value varies based on the units used for pressure, volume, and temperature.
- What are some limitations of the Ideal Gas Equation?
- While the Ideal Gas Equation is a useful approximation for many gases under various conditions, it has limitations. For instance, it assumes that gas particles have no volume and do not attract or repel each other, which may not hold true for real gases under high pressures or low temperatures.
- How does the Ideal Gas Equation relate to Boyle’s, Charles’s, and Avogadro’s Laws?
- The Ideal Gas Equation incorporates Boyle’s Law (PV = k), Charles’s Law (V ∝ T), and Avogadro’s Law (V ∝ n) as special cases when specific variables are held constant. These laws describe the behavior of ideal gases under different conditions.
- What is the physical significance of the Ideal Gas Constant (R)?
- The value of the Ideal Gas Constant, R, represents the amount of energy absorbed or released by one mole of an ideal gas when its temperature changes by 1 Kelvin at standard conditions. It serves as a universal parameter for all gases.
- How is the Ideal Gas Equation used in practical applications?
- The Ideal Gas Equation is used extensively in various fields, including chemistry, physics, and engineering. It helps in predicting and understanding the behavior of gases under different conditions, such as in chemical reactions, industrial processes, and the design of gas-containing systems.
Summary: Ideal Gas Equation Tutorial
The Ideal Gas Equation tutorial provides an in-depth understanding of ideal gases, their behavior, and the principles governing them. It begins by defining an ideal gas as a theoretical concept composed of point particles interacting through elastic collisions, allowing for analysis under statistical mechanics.
The tutorial delves into the fundamental Ideal Gas Laws discovered by Boyle and Charles, which describe the relationship between pressure, volume, and temperature for ideal gases. Boyle’s Law states an inverse proportionality between pressure and volume at constant temperature, while Charles’s Law establishes a direct proportionality between volume and temperature at constant pressure.
The cornerstone of the tutorial is the Ideal Gas Equation, PV = nRT, which encapsulates the behavior of ideal gases. It explains the formulation of the equation by combining Boyle’s, Charles’s, and Avogadro’s laws, providing a comprehensive understanding of gas behavior under various conditions.
Additionally, the tutorial elucidates the significance of the Ideal Gas Constant, R, in determining the value of the equation and its universal application across different gases.
In conclusion, the Ideal Gas Equation tutorial equips learners with the knowledge and principles necessary to comprehend the behavior of ideal gases and their practical implications in various scientific and engineering fields.
