Covalent Bond Definition
A covalent bond is established when elements from Group-13 to Group-17 react, engaging in the mutual sharing of valence shell electrons. This bond, resulting from the shared electrons, is known as a covalent bond.
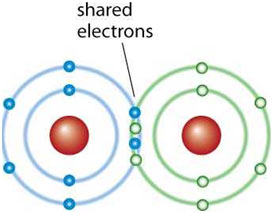
In the process of covalent bond formation, significant changes in energy occur. As two atoms approach each other, attractive forces arise between the electrons of one atom and the nucleus of the other. Simultaneously, repulsive forces manifest between the electrons and nuclei of both atoms.
The decisive factor in bond formation is the dominance of attractive forces as the distance between the two atoms decreases. This phenomenon is exemplified in the creation of chemical bonds seen in hydrogen, chlorine, nitrogen, and oxygen gases.
- 1) Types of covalent bonds
- 2) Single Covalent bond
- 3) Double Covalent bond
- 4) Triple Covalent Bond
- 5) Dative Covalent or Coordinate Covalent Bond
- 6) Polar and Non-polar Covalent Bond
- 7) Frequently Asked Questions (FAQs)
- 8) Multiple-Choice Questions (MCQs) with Answers
- 9) Summary: Covalent Bond, Types, Polar and Non-polar Bond
- 10) You may also like to learn:
Types of covalent bonds
The covalent bond is formed by the shared of electrons between two atoms. The electrons that pair to form a chemical bond are called ‘bond pair’ electrons. Depending upon the number of bond pairs, a covalent bond is classified into the following three types:
Single Covalent bond
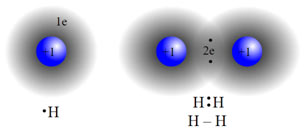
When one electron is contributed by each bonded atom, one bond pair is formed and it forms a single covalent bond. While drawing the structure of such molecules the single bond pair is shown by a line between those two atoms (___). A few examples of molecules with single covalent bonds are hydrogen (H2), chlorine (cl2), hydrochloric acid (HCl), etc.
Double Covalent bond
When each bonded atom contributes two electrons, two bond pairs are shared and a double covalent bond is formed. These bond pairs are indicated as a double line between those atoms in the structure of such molecules. The particles like oxygen (O2)gas and ethene (C2H4) reveal such type of double covalent bonds.

Triple Covalent Bond
When each bonded atom contributes three electrons, three bond pairs are associated with bond development. This type is called a triple covalent bond. Three small lines are utilized to show these three pairs of electrons in between those atoms in the molecules of such compounds. The examples of molecules having triple covalent bonds are nitrogen (N2) and ethyne (C2H2).

By this mutual sharing of valence shell electrons, each of the contributing atoms attains the ‘Octet’ or nearest noble gas electronic configuration.
Dative Covalent or Coordinate Covalent Bond
Coordinate covalent or dative covalent bonding is a type of covalent bonding in which the bond set of electrons is donated by one bonded atom only. The atom which contributes the electron pair is called the donor and the atom which accepts the electron pair is called the acceptor. A little arrow is generally used to suggest the atom and set of electrons being donated. The head of the arrow is towards the acceptor atom.
The non-bonded electron pair available on an atom, like the one available on nitrogen in ammonia, (NH3) is called a lone pair. When a proton (H+) approaches a molecule with an only set of electrons, that only set is contributed to H+, and a coordinate covalent bond is formed, e.g. formation of ammonium radical (NH4+).
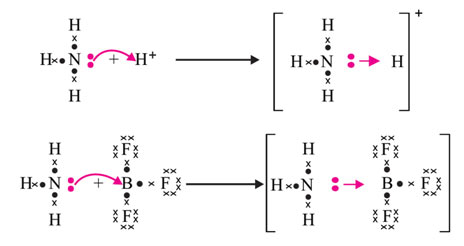
In the formation of BF3 (boron trifluoride) molecule, three valence electrons of boron atom (Z= 5) pair with three electrons, one from each 3 fluorine atoms. The boron atom even after this sharing of electrons (covalent bond formation), remains short or deficit of 2 electrons in its outer shell. Now if a molecule with a lone pair approaches this molecule, it accepts a lone set from that donor and forms a coordinate covalent bond. The only pair on the nitrogen of the ammonia molecule makes it a good donor molecule to form a coordinate covalent bond.
Polar and Non-polar Covalent Bond
If a covalent bond is formed between two similar atoms (homo-atoms), the shared pair of electrons is attracted by both the atoms equally. Such kind of bond is called a nonpolar covalent bond. These bonds are formed by equal sharing of electron set between the two bonding atoms. This type of bond is called a pure covalent bond. For instance, bond formation in H2 and CI2.
If the covalent bond is formed between two different kinds of atoms (heteroatoms) then the bond pair of electrons will not be brought in similarly by the bonded atoms. One of the atoms will attract the bond pair of electrons more strongly than the other one. This atom(element) will be called as more electronegative.
When there is a difference of electronegativity between two covalently bonded atoms, there will be an unequal attraction for the bond pair of electrons between such atoms.
It will result in the development of a polar covalent bond. The distinction between the electronegativities of hydrogen and chlorine is 1.0. As the electronegativity of chlorine is more, it draws in the shared pair of electrons towards itself with a greater force. A partial negative charge is therefore produced on chlorine and in turn a partial positive charge on hydrogen due to electronegativity difference. It develops polarity in the bond and is called polar covalent bond.
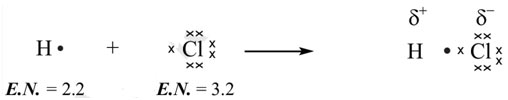
The delta () sign shows partial positive or partial negative charge that is developed due to unequal sharing of shared pairs or bonded pairs of electrons. The substances resulting from polar covalent bonds are called polar compounds. For example: water, hydrogen fluoride, and hydrogen chloride.

By utilizing electronegativity values, it is possible to anticipate whether a chemical bond will be ionic or covalent in nature. A bond formed between elements of high electronegativity (halogen group) and components of low electronegativity (alkali metals) is ionic in nature.
There is a complete transfer of electrons between them. The bond between elements of similar electronegativities will be covalent in nature as the bond between carbon and hydrogen in methane, or nitrogen and hydrogen in ammonia. If the difference of electronegativities between two elements is more than 1.7 the bond between them will be primarily ionic bond and if it is less than 1.7, the bond between two atoms will be mainly covalent.
Frequently Asked Questions (FAQs)
1. What is a Covalent Bond?
- Definition: A covalent bond is formed when atoms from Group-13 to Group-17 share valence shell electrons, resulting in mutual electron sharing.
2. What are the Types of Covalent Bonds?
- Single Covalent Bond: Formed by the sharing of one electron from each bonded atom.
- Double Covalent Bond: Formed by the sharing of two electrons from each bonded atom.
- Triple Covalent Bond: Formed by the sharing of three electrons from each bonded atom.
3. Can you provide examples of Molecules with Covalent Bonds?
- Single Covalent Bond: Examples include hydrogen (H₂), chlorine (Cl₂), and hydrochloric acid (HCl).
- Double Covalent Bond: Oxygen gas (O₂) and ethene (C₂H₄) are examples.
- Triple Covalent Bond: Nitrogen gas (N₂) and ethyne (C₂H₂) exhibit triple covalent bonds.
4. What is Dative Covalent or Coordinate Covalent Bonding?
- Definition: It is a type of covalent bonding where one atom donates a pair of electrons (lone pair) to another, forming a coordinate covalent bond.
5. Can you explain the concept of Polar and Non-polar Covalent Bonds?
- Nonpolar Covalent Bond: Formed when the shared pair of electrons is equally attracted by similar atoms, as seen in H₂ and Cl₂.
- Polar Covalent Bond: Occurs when there’s an unequal sharing of electrons between different atoms, creating partial positive and negative charges. Examples include water (H₂O) and hydrogen fluoride (HF).
6. How is Electronegativity related to Covalent Bonds?
- Electronegativity: It measures an atom’s ability to attract shared electrons. A higher electronegativity difference between bonded atoms results in a polar covalent bond, while a smaller difference leads to a nonpolar covalent bond.
7. What is the significance of the delta (δ) sign in Polar Covalent Bonds?
- Delta (δ) Sign: Represents partial positive or partial negative charges due to unequal sharing of electrons, indicating polarity in the bond.
8. How do you determine whether a bond is Ionic or Covalent using Electronegativity?
- Ionic Bond: Formed between elements with a large electronegativity difference (greater than 1.7).
- Covalent Bond: Occurs when the electronegativity difference is less than 1.7 between bonded atoms.
9. Can you provide examples of Polar Compounds?
- Examples: Water (H₂O), hydrogen fluoride (HF), and hydrogen chloride (HCl) are polar compounds resulting from polar covalent bonds.
10. Why is Covalent Bonding important in achieving Electron Configuration?
- Achieving Octet: Covalent bonding involves sharing electrons, allowing each participating atom to attain an octet or the nearest noble gas electronic configuration.
Multiple-Choice Questions (MCQs) with Answers
- What is a covalent bond?
- A. Ionic bond
- B. Metallic bond
- C. Bond formed by electron sharing
- D. Van der Waals bond
- Answer: C
- Which elements participate in covalent bonding according to the given passage?
- A. Group-1 to Group-12
- B. Group-13 to Group-17
- C. Group-18 only
- D. Transition metals
- Answer: B
- What forces are involved in the development of a covalent bond?
- A. Magnetic forces
- B. Gravitational forces
- C. Attractive and repulsive forces between electrons and nuclei
- D. Electrostatic forces between protons
- Answer: C
- In covalent bonding, when does a chemical bond form between two atoms?
- A. When repulsive forces dominate
- B. When attractive forces dominate
- C. When atoms collide
- D. When atoms move apart
- Answer: B
- Which of the following is an example of a molecule with a single covalent bond?
- A. Oxygen gas (O₂)
- B. Chlorine gas (Cl₂)
- C. Hydrogen chloride (HCl)
- D. Methane (CH₄)
- Answer: D
- How is a double covalent bond represented in molecular structures?
- A. A single line (___)
- B. A double line (=)
- C. Two dots (..)
- D. Two arrows (->)
- Answer: B
- Which molecules exhibit triple covalent bonds?
- A. Oxygen gas (O₂)
- B. Nitrogen gas (N₂)
- C. Ethene (C₂H₄)
- D. Hydrochloric acid (HCl)
- Answer: B
- What is the term for the type of covalent bonding where electrons are donated by one bonded atom only?
- A. Double bond
- B. Dative covalent or coordinate covalent bond
- C. Triple bond
- D. Metallic bond
- Answer: B
- What is a lone pair in covalent bonding?
- A. A pair of shared electrons
- B. A pair of electrons donated by an atom
- C. A pair of unshared electrons
- D. A pair of protons
- Answer: C
- In the formation of BF₃, how many electrons does boron contribute to bond with fluorine?
- A. One
- B. Two
- C. Three
- D. Four
- Answer: C
- What is the result of a nonpolar covalent bond?
- A. Unequal sharing of electrons
- B. No sharing of electrons
- C. Equal sharing of electron pairs
- D. Formation of ions
- Answer: C
- How is a polar covalent bond characterized?
- A. By equal sharing of electrons
- B. By unequal sharing of electrons
- C. By complete transfer of electrons
- D. By metallic bonding
- Answer: B
- What does the delta (δ) sign represent in polar covalent bonds?
- A. Positive charge
- B. Negative charge
- C. Partial positive or partial negative charge
- D. Full positive charge
- Answer: C
- Which substances are examples of polar compounds?
- A. Methane, water, and hydrogen chloride
- B. Oxygen gas, ethene, and hydrochloric acid
- C. Hydrogen, chlorine, and nitrogen
- D. Oxygen gas, hydrogen fluoride, and nitrogen
- Answer: D
- How is the nature of a chemical bond determined using electronegativity values?
- A. By counting electrons
- B. By analyzing nuclear charge
- C. By calculating bond length
- D. By comparing electronegativity differences
- Answer: D
Summary: Covalent Bond, Types, Polar and Non-polar Bond
The tutorial begins by defining a covalent bond as the mutual sharing of valence shell electrons between elements of Group-13 to Group-17. It emphasizes the significance of attractive and repulsive forces during covalent bond formation, highlighting examples like hydrogen, chlorine, nitrogen, and oxygen gases.The three types of covalent bonds are introduced based on the number of shared electrons:
- Single Covalent Bond: Formed by the sharing of one electron from each bonded atom, represented by a single line in molecular structures.
- Double Covalent Bond: Involves the sharing of two electrons from each bonded atom, symbolized by a double line in molecular diagrams.
- Triple Covalent Bond: Formed when each bonded atom contributes three electrons, illustrated by three lines connecting the atoms.
The concept of dative covalent or coordinate covalent bonding is explained, where one atom donates a bond pair of electrons to another. The interaction of lone pairs, illustrated in the formation of ammonium radical (NH₄⁺) and BF₃ molecule, is detailed.
The section concludes with the distinction between polar and nonpolar covalent bonds. Nonpolar covalent bonds occur between similar atoms, with equal electron sharing, while polar covalent bonds involve heteroatoms, leading to unequal electron sharing. The role of electronegativity in determining bond nature is highlighted, and the tutorial concludes by mentioning that a difference of electronegativity greater than 1.7 results in an ionic bond, while a difference less than 1.7 indicates a mainly covalent bond. The tutorial provides a comprehensive understanding of covalent bonding and its variations.
