What is Steel
Steel is an alloy of iron containing 0.25 to 2.5% of carbon as well as traces of S, P, Si, and Mn.
Classification of Steel
Mild Steel (0.1 – 0.2 % C)
It is relatively soft, flexible and ductile, and can be shaped (formed by hammering and also pushing while hot). It is used in making tubes, nuts, screws, bars, and boiler plates.
Medium Carbon Steel (0.2 – 0.7 % C)
It is harder than mild steel. It is also malleable and ductile. It is utilized in making rails, axles, and castings.
High Carbon Steel (0.7 -1.5 % C)
It is hard and can be shaped when having less than 1.0% carbon. Steel having more than 1.0% carbon cannot be forged. It is utilized to make hammers, faucets, cutting tools, machine tools, hard steel parts of equipment, and all type of engines.
Steel is intermediate in carbon content between cast iron and wrought iron. It can be produced from:
- Cast iron by removing some carbon along with sulphur, phosphorus, and silicon.
- By adding a small amount of carbon to wrought iron, then including some unique constituents, e.g., tungsten, chromium, vanadium, molybdenum, manganese, nickel and cobalt which pass on desired properties to the steel.
Presently most of the steel is produced from cast iron.
Manufacturing of Steel
Steel can be produced by following processes.
- Open hearth process (using cast iron, wrought iron, or steel scrap)
- Bessemer’s process (utilizing cast iron only)
Open Hearth Process
This is one of the most modern-day techniques for the manufacturing of steel. It is performed in an open-hearth heating system. This furnace has a low roof covering to deflect the warm gases and fires downward to melt the charge. The open-hearth furnace works on the regenerative principle of heat economy.
Open hearth process is of two kinds
- Furnace with acidic lining like SiO2 is made use of when the contaminations are Mn, Si, etc.
- Furnace with basic lining like dolomite (Ca O, MgO) is utilized when the impurities are P and S, etc.
Process
- A combination of cast iron, scrap steel, and quick lime is charged into the furnace.
- At about 1600 °C – Si, Mn, C, S, and P are burnt out and eliminated according to the following reactions.
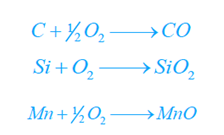
CO escapes in the flue gases. Silica (SiO2) incorporates with CaO, MnO, and FeO to develop silicates (slag) that float on the surface of the molten metal.
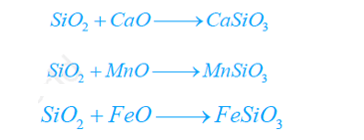
Phosphorus as well as sulphur react with Fe2O3 to produce P2O5 and SO2.
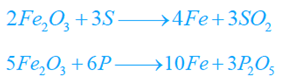
These oxides react with calcium oxide to form slag.
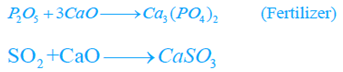
Samples are taken at periods as well as the percent of carbon in the steel is checked regularly. When this is reduced to about 0.1%, the calculated weight of ferromanganese (Fe, Mn, C) is added.
- Manganese desulphurizes the steel. Carbon increases the carbon components to the required values. After giving time for blending, small amount of ferromanganese is added, and the charge is permitted to face moulds where it strengthens to ingots.
- The entire process takes about 10 hrs. Slag has calcium phosphate. It is ground to powder and marketed as a fertilizer.
Bessemer’s Process
The furnace used in this process is called Bessemer’s Converter which is a pear-shaped vessel made from steel plates. Near the bottom, the converter is given with a number of holes whereby hot air can be introduced.

The converter is hung on a main axis to ensure that it can be tilted in any preferred setting for feeding and also pouring out the finished materials.
Molten pig or cast iron (25 to 30 tons) from the blast heating system is fed right into the converter and hot air blast is infused via the perforated base. This oxidizes carbon, silicon, and manganese.
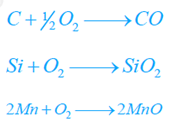
These oxides create a slag of MnSiO3.The heat evolved during the oxidation suffices to maintain iron in the molten state.
![]()
Carbon Monoxide produced burns at the mouth of the converter with a blue fire. Iron is partially oxidized to ferric oxide (Fe2O3) which also extracts carbon from cast iron to form CO.
Within 10 to 15 mins the flame due to Carbon Monoxide subsides indicating that the carbon is totally oxidized. At this phase, ferromanganese is added to fix the proportion of carbon to obtain the desired top qualities. A blast of air is continued for a moment to make sure of detailed mixing. The addition of Mn imparts enhanced firmness and tensile strength.
In order to remove entrapped bubbles of gases (blow holes), such as O2, N2, CO2, small amount of aluminum or ferrosilicon is also added. Lightweight aluminum removes nitrogen as nitride.
![]()
At the end of the operation, the molten steel is poured out right into moulds for spreading. Such casting is devoid of any defect.
MCQs about Steel
- Which of the following elements is NOT typically present in steel?
- A) Carbon
- B) Silicon
- C) Oxygen
- D) Manganese
- Answer: C) Oxygen
- What is the carbon content range of Mild Steel?
- A) 0.1 – 0.2%
- B) 0.2 – 0.7%
- C) 0.7 – 1.5%
- D) 1.5 – 2.5%
- Answer: A) 0.1 – 0.2%
- Which type of steel is harder than Mild Steel but still malleable and ductile?
- A) High Carbon Steel
- B) Medium Carbon Steel
- C) Stainless Steel
- D) Alloy Steel
- Answer: B) Medium Carbon Steel
- What process is used for the manufacturing of steel in an open-hearth heating system?
- A) Bessemer’s Process
- B) Open Hearth Process
- C) Electric Arc Furnace Process
- D) Oxygen Process
- Answer: B) Open Hearth Process
- Which lining is used in the Open Hearth furnace when impurities are mainly phosphorus and sulfur?
- A) Basic lining
- B) Acidic lining
- C) Alkaline lining
- D) Dolomite lining
- Answer: A) Basic lining
- What is the primary purpose of ferromanganese addition during the Open Hearth process?
- A) Increase ductility
- B) Decrease carbon content
- C) Desulphurize the steel
- D) Enhance tensile strength
- Answer: C) Desulphurize the steel
- Which type of vessel is used in Bessemer’s process for steel manufacturing?
- A) Converter
- B) Furnace
- C) Crucible
- D) Kiln
- Answer: A) Converter
- What is the composition of the slag produced in Bessemer’s process?
- A) MnSiO2
- B) MnSiO3
- C) FeO
- D) Fe2O3
- Answer: B) MnSiO3
- What is the purpose of adding ferrosilicon or aluminum in Bessemer’s process?
- A) Increase ductility
- B) Remove impurities
- C) Enhance hardness
- D) Control carbon content
- Answer: B) Remove impurities
- How long does it typically take for the flame due to Carbon Monoxide to subside in Bessemer’s process?
- A) 5 to 10 minutes
- B) 10 to 15 minutes
- C) 15 to 20 minutes
- D) 20 to 25 minutes
- Answer: B) 10 to 15 minutes
- What is the primary purpose of introducing hot air into the converter during Bessemer’s process?
- A) To reduce temperature
- B) To oxidize carbon and silicon
- C) To remove sulfur
- D) To enhance malleability
- Answer: B) To oxidize carbon and silicon
- What is the primary constituent that allows steel to be shaped when it contains less than 1.0% carbon?
- A) Silicon
- B) Manganese
- C) Chromium
- D) Nickel
- Answer: B) Manganese
- Which of the following processes utilizes only cast iron for steel production?
- A) Open Hearth Process
- B) Electric Arc Furnace Process
- C) Bessemer’s Process
- D) Oxygen Process
- Answer: C) Bessemer’s Process
- What is the typical carbon content range of High Carbon Steel?
- A) 0.1 – 0.2%
- B) 0.2 – 0.7%
- C) 0.7 – 1.5%
- D) 1.5 – 2.5%
- Answer: C) 0.7 – 1.5%
- What is the primary purpose of dolomite lining in the Open Hearth furnace?
- A) To reduce temperature
- B) To oxidize carbon and silicon
- C) To remove sulfur
- D) To enhance malleability
- Answer: C) To remove sulfur
- Which process is known for utilizing the regenerative principle of heat economy?
- A) Bessemer’s Process
- B) Electric Arc Furnace Process
- C) Open Hearth Process
- D) Oxygen Process
- Answer: C) Open Hearth Process
- Which element is NOT typically added during the Open Hearth process for steel production?
- A) Manganese
- B) Chromium
- C) Tungsten
- D) Vanadium
- Answer: B) Chromium
- What is the primary purpose of adding ferromanganese during the Open Hearth process?
- A) Increase ductility
- B) Decrease carbon content
- C) Desulphurize the steel
- D) Enhance tensile strength
- Answer: C) Desulphurize the steel
- Which process produces slag that is marketed as a fertilizer?
- A) Bessemer’s Process
- B) Electric Arc Furnace Process
- C) Oxygen Process
- D) Open Hearth Process
- Answer: D) Open Hearth Process
- What is the primary function of adding quick lime in the Open Hearth process?
- A) Increase carbon content
- B) Reduce temperature
- C) Remove impurities
- D) Enhance hardness
- Answer: C) Remove impurities
- Which of the following is NOT a type of steel classification mentioned in the passage?
- A) Stainless Steel
- B) Alloy Steel
- C) Cast Iron Steel
- D) Mild Steel
- Answer: C) Cast Iron Steel
- What is the primary purpose of the basic lining in the Open Hearth furnace?
- A) To remove sulfur and phosphorus
- B) To increase carbon content
- C) To enhance malleability
- D) To oxidize carbon and silicon
- Answer: A) To remove sulfur and phosphorus
- Which of the following elements is primarily responsible for desulphurizing steel during the Open Hearth process?
- A) Silicon
- B) Manganese
- C) Carbon
- D) Aluminum
- Answer: B) Manganese
- Which process utilizes hot air blast to oxidize carbon, silicon, and manganese?
- A) Electric Arc Furnace Process
- B) Bessemer’s Process
- C) Oxygen Process
- D) Open Hearth Process
- Answer: B) Bessemer’s Process
Summary:
In this tutorial, we explored the world of steel, covering its classification and manufacturing processes. Steel, an alloy of iron with carbon and various other elements, comes in different grades based on its carbon content. These grades include Mild Steel, Medium Carbon Steel, and High Carbon Steel, each with distinct properties and applications.
Steel can be manufactured through various processes, two of which are prominently discussed: the Open Hearth Process and Bessemer’s Process. The Open Hearth Process involves heating a mixture of cast iron, scrap steel, and quick lime in a furnace, with subsequent reactions leading to the removal of impurities and the adjustment of carbon content. On the other hand, Bessemer’s Process utilizes a converter to oxidize carbon, silicon, and manganese from molten pig iron, followed by the addition of ferromanganese to attain desired qualities.
Both processes demonstrate sophisticated techniques for steel production, showcasing the industry’s advancements in metallurgy and engineering. Understanding these processes sheds light on the complex journey from raw materials to the versatile material we know as steel.

