Water is important for all life forms. It is an inorganic, colour-less and almost tasteless chemical substance with the molecular formula H2O.
The oceans contain 97% of the total Earth’s water.
From which only 0.2% of water is safe and clean for drinking and other domestic purposes. The maximum density of water is 1 gcm-3 at 4 °C. Water has the ability to absorb heat with minimum change in its own temperature, this is called the specific heat capacity of water.
Water has great solvent properties due to its polarity.
The water molecule ionizes to form H+ and OH– ions. At 25 ° C, the concentration of each of H+ and OH- ions in distilled water is 10-7 mole/litre.
Water is an effective lubricant that provides safety to organs in the body by providing fluid cushion and prevents from trauma or injury.
Water has many uses in domestic and industrial life. Also used in farming and agriculture. Thus, the vital component of life on Earth.
- 1) Water – The Life-Giving Fluid
- 2) Molecular Formula
- 3) Water on Earth
- 4) Properties of Water
- 5) Importance of Water
- 6) Solvent properties
- 7) Specific Heat Capacity
- 8) Heat of vaporization
- 9) Ionization of water
- 10) Protection
- 11) Uses of Water
- 12) Multiple Choice Questions (MCQs) on Water
- 13) Frequently Asked Questions (FAQs)
- 14) Wrap up
- 15) You may also like to learn:
Water – The Life-Giving Fluid
Definition:
Water is an inorganic, transparent, tasteless, odor-free, and nearly colourless chemical substance, which is the primary constituent of Earth’s hydrosphere and the fluids of all living organisms.
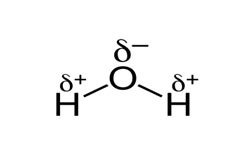
Molecular Formula
Water on Earth
The oceans contain about 97% of Earth’s water. The remaining of the water is in the form of glaciers, ice caps, groundwater and inland water (rivers, lakes, streams). It is likewise present in the atmosphere in the form of water vapours. Seawater is unsuited for drinking and farming purposes due to high portion of dissolved salts.
Only 0.2% of the overall water on the Earth is safe and clean, i.e. fit for drinking purposes.

Properties of Water
Water is made up of two components: oxygen and hydrogen. One atom of oxygen combines with two atoms of hydrogen to form one molecule of water. Distilled water is a clear, colourless, odourless and tasteless liquid with the following properties:
- It is neutral to litmus.
- Its freezing point is 0 ° C and the boiling point is 100 ° C at sea level.
- Its optimum density is 1 g cm-3 at 4 °C.
- It is an outstanding solvent for ionic in addition to molecular substances.
- It has an abnormally high heat capacity of about 4.2 Jg-1K-1, which is about six times greater than that of land. This specific property of water is responsible for keeping the Earth’s temperature level within limits. Otherwise, day time temperature would have been extremely high to bear and night time temperature level would have been too low to freeze everything.
- It has high surface area tension. This distinct property of water is accountable for its high capillary action. Capillary action is the procedure by which water rises up from the roots of plants to leaves. This procedure is important for the survival of the land plants.
Importance of Water
Water is the medium of life. It is the most plentiful substance in all organisms. It varies from 65 to 89 per cent in different organisms. Human tissues consist of about 20 per cent of water in bone cells and 85% in brain cells. Almost all actions of a cell take place in the existence of water. It likewise participates in numerous biochemical reactions such as hydrolysis of macromolecules. It is also used as a raw material in photosynthesis.
Solvent properties
Due to its polarity, water is an excellent solvent for polar substances. Ionic compounds when dissolved in water, dissociate into positive and negative ions. Non-ionic substances having charged groups in their molecules are distributed in the water.
When in solution, ions and molecules move arbitrarily and are in a more favourable state to react with other particles and ions. It is because of this characteristic of water that almost all reactions in cells occur in liquid media.
In cells, all chemical reactions are catalyzed by enzymes which operate in a liquid environment. Nonpolar natural particles, such as fats, are insoluble in water and help to keep membranes which make compartments in the cell.
Specific Heat Capacity
Water has the ability to take in the heat with a minimum of change in its own temperature level. The specific heat capacity of water – the number of calories required to raise the temperature of 1g of water from 15 to 16 ° C is 1.0. This is because much of the energy is used to break hydrogen bonds. Water hence works as temperature level stabilizer for organisms in the environment and hence secures living organisms against unexpected thermal changes.
Heat of vaporization
Water takes in much heat as it alters from liquid to gas. The heat of vaporization is expressed as calories taken in per gram vaporized. The specific heat of vaporization of water is 574 Kcal/kg, which plays an important function in the maintenance of heat produced by oxidation.
It likewise offers a cooling effect to plants when water is transpired, or to animals when water is perspired. Evaporation of just two ml out of one litre of water lowers the temperature of the remaining 998 ml by 1 ° C.
Ionization of water
The water moleculeionizes to form H+ and OH– ions:

This reaction is reversible however a balance is preserved. At 25 ° C, the concentration of each of H+ and OH- ions in distilled water is 10-7 mole/litre. The H+ and OH– ions affect, and take part in a number of the reactions that take place in cells.
Protection
Water is an effective lubricant that provides safety against damage arising from friction. For instance, tears protect the surface of the eye from the rubbing of eyelids, water likewise forms a fluid cushion around organs that assists to protect them from trauma.
Uses of Water

Water is stored in numerous parts of the world however not evenly distributed all over the earth. It is a universal solvent. Different sources of water are– sea, lake, rain, well, stream, borehole and pond. It is used for washing, drinking, generating electrical energy and so on. Here are the various uses of water.
Domestic uses of water
15 % of water is consumed for domestic purpose. Water is utilized for drinking, bathing, cooking food and cleaning utensils, clothes, fruits, veggies and brushing teeth.
Water usage for farming
Agriculture is the largest consumer of water. About 70% of water is used for irrigation. Water is essential for gardening, farming and fisheries. Plants need water to grow. Throughout the procedure of photosynthesis, they take in water. To yield crops, fruits, flowers, veggies they require adequate water, manure and oxygen.
Industrial uses of water
Industrial water is used for cleaning, cooling, processing, transferring, diluting or fabricating of a product. The maximum quantity of water is utilized in the production of chemical, paper and food.
Other uses are– it is utilized in transportation, manufacturing, hydroelectric power, elimination of body wastes.
Multiple Choice Questions (MCQs) on Water
1. What is the molecular formula of water?
- A) HO
- B) H2O
- C) O2
- D) CO2
Answer: B) H2O
2. What percentage of the Earth’s water is found in oceans?
- A) 50%
- B) 75%
- C) 97%
- D) 100%
Answer: C) 97%
3. What is the maximum density of water?
- A) 0.5 g/cm³
- B) 1 g/cm³ at 4 °C
- C) 2 g/cm³
- D) 0.8 g/cm³
Answer: B) 1 g/cm³ at 4 °C
4. What is the specific heat capacity of water compared to land?
- A) About the same
- B) Six times greater
- C) Half
- D) Double
Answer: B) Six times greater
5. What is the heat of vaporization of water expressed as calories per gram vaporized?
- A) 100 Kcal/g
- B) 574 Kcal/kg
- C) 200 Kcal/g
- D) 400 Kcal/kg
Answer: B) 574 Kcal/kg
6. How much of the Earth’s water is safe and clean for drinking purposes?
- A) 50%
- B) 0.2%
- C) 10%
- D) 1%
Answer: B) 0.2%
7. What ions does water ionize into?
- A) H+ and OH–
- B) Na+ and Cl–
- C) CO3^2– and HCO3–
- D) Fe3+ and SO4^2–
Answer: A) H+ and OH–
8. What is the primary constituent of Earth’s hydrosphere and the fluids of all living organisms?
- A) Methane
- B) Water
- C) Oxygen
- D) Nitrogen
Answer: B) Water
9. What is the primary use of water in agriculture?
- A) Drinking
- B) Bathing
- C) Irrigation
- D) Cooking
Answer: C) Irrigation
10. What property of water is responsible for its high capillary action?
- A) High boiling point
- B) High surface tension
- C) Low density
- D) Low freezing point
Answer: B) High surface tension
11. What is the freezing point of water at sea level?
- A) -10 °C
- B) 0 °C
- C) 10 °C
- D) 20 °C
Answer: B) 0 °C
12. How does water act as a temperature stabilizer in the environment?
- A) By increasing temperature rapidly
- B) By decreasing temperature rapidly
- C) By maintaining a stable temperature due to high heat capacity
- D) By resisting temperature changes
Answer: C) By maintaining a stable temperature due to high heat capacity
13. What is the primary role of water in biochemical reactions within cells?
- A) Catalyzing reactions
- B) Providing energy
- C) Preventing reactions
- D) Stabilizing pH
Answer: A) Catalyzing reactions
14. How does water provide protection against damage from friction?
- A) By forming a fluid cushion
- B) By increasing friction
- C) By reducing temperature
- D) By solidifying
Answer: A) By forming a fluid cushion
15. What percentage of water is used for industrial purposes?
- A) 10%
- B) 25%
- C) 50%
- D) 70%
Answer: D) 70%
16. What is the primary reason seawater is unsuitable for drinking and farming?
- A) High temperature
- B) Low oxygen content
- C) High dissolved salt content
- D) Presence of pollutants
Answer: C) High dissolved salt content
17. Which of the following is NOT a domestic use of water?
- A) Drinking
- B) Cooking
- C) Cooling industrial equipment
- D) Bathing
Answer: C) Cooling industrial equipment
Frequently Asked Questions (FAQs)
1. What is the molecular formula of water?
- Answer: The molecular formula of water is H2O.
2. What percentage of Earth’s water is found in the oceans?
- Answer: About 97% of Earth’s water is found in the oceans.
3. Why is seawater unsuitable for drinking and farming?
- Answer: Seawater is unsuitable due to its high concentration of dissolved salts.
4. At what temperature does water have its maximum density?
- Answer: Water has its maximum density at 4 °C.
5. How does water act as a temperature stabilizer in the environment?
- Answer: Water’s high specific heat capacity allows it to absorb heat with minimal temperature change, stabilizing environmental temperatures.
6. What is the significance of water’s high surface tension?
- Answer: High surface tension is responsible for capillary action, vital for water transport in plants.
7. What percentage of human tissues is composed of water?
- Answer: Human tissues vary, but they generally contain about 20% water in bone cells and 85% in brain cells.
8. How does water participate in biochemical reactions within cells?
- Answer: Water acts as a medium for biochemical reactions, including the hydrolysis of macromolecules and as a raw material in photosynthesis.
9. Why is water considered an excellent solvent?
- Answer: Water’s polarity makes it an excellent solvent for polar substances, causing ionic compounds to dissociate into ions.
10. What role does water play in protecting organs in the body?
- Answer: Water acts as an effective lubricant, forming a fluid cushion around organs, protecting them from trauma or injury.
11. What is the specific heat capacity of water?
- Answer: The specific heat capacity of water is about 4.2 Jg-1K-1.
12. How does water contribute to the cooling effect in plants and animals?
- Answer: Water’s high heat of vaporization provides a cooling effect during processes like transpiration in plants and perspiration in animals.
13. How does water ionize, and what is the concentration of H+ and OH- ions in distilled water?
- Answer: Water ionizes to form H+ and OH- ions. In distilled water at 25 °C, the concentration of each ion is 10-7 mole/litre.
14. What are the various domestic uses of water?
- Answer: Domestic uses include drinking, bathing, cooking, and cleaning utensils, clothes, fruits, vegetables, and brushing teeth.
15. Why is agriculture the largest consumer of water?
- Answer: About 70% of water is used for irrigation in agriculture, essential for the growth of crops, fruits, flowers, and vegetables.
16. How is water utilized in industrial processes?
- Answer: Industrial water is used for cleaning, cooling, processing, transferring, diluting, or fabricating products, with chemical, paper, and food production being major consumers.
17. What are some other miscellaneous uses of water?
- Answer: Water is used in transportation, manufacturing, hydroelectric power, and the elimination of body wastes.
Wrap up
Water, with the chemical formula H2O, is a crucial element for all life forms. This tutorial explores various aspects of water, covering its definition, molecular formula, presence on Earth, properties, and importance in domestic, industrial, and agricultural contexts.
The oceans hold 97% of Earth’s water, but only 0.2% is safe for drinking. Water’s unique properties, such as its maximum density at 4 °C and high heat capacity, contribute to stabilizing environmental temperatures. Water’s solvent properties, driven by its polarity, make it essential for biochemical reactions in living organisms.
The tutorial emphasizes water’s role as a life-giving fluid, detailing its importance as a medium for life, a lubricant offering protection against trauma, and a participant in biochemical reactions. It explores water’s uses in domestic settings, agriculture, and industry, where it serves purposes like cleaning, cooling, and manufacturing.
In conclusion, water’s significance extends beyond its chemical properties; it is a universal solvent, a temperature stabilizer, and a fundamental element for life on Earth. Understanding water’s diverse uses and properties is vital for appreciating its indispensable role in sustaining life and supporting various human activities.

