Sodium and Preparation of Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal.
- Sodium is an alkali metal, being in group 1 of the table of elements. Its only steady isotope is ²³ Na.
- The free metal does not take place in nature and should be prepared from compounds. Salt is the 6th most common element in the world and makes up 2.6% of the Earth’s crust.
- The most common compound is sodium chloride.
- This extremely soluble salt has seeped into the oceans over the lifetime of the world, however many salt beds or ‘lakes’ are found where ancient seas have evaporated.
- It is likewise discovered in numerous minerals including cryolite, zeolite, and sodalite.
- Because sodium is so reactive it is never discovered as a metal in nature.
- Sodium metal is produced by electrolysis of dry molten sodium chloride.
Chemical Preparation of Salt by Downs Cell
Most salt metal is produced by the electrolysis of fused sodium chloride. Given that the melting point of sodium chloride is 801 ° C, some calcium chloride is contributed to decrease its melting point and to permit the furnace to run at about 600° C.
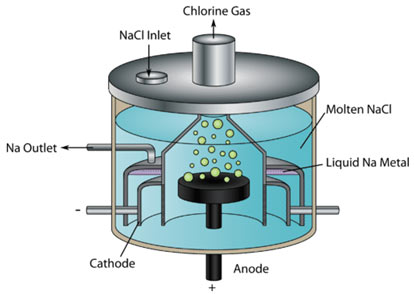
In the electrolytic cell, the large block of graphite at the center is the anode, above which there is a dome for the collection of chlorine. The cathode is a circular bar of copper or iron that surrounds the anode but is separated from it by an iron screen, which is terminated in a gauze. The arrangement permits the electric current to pass freely but prevents sodium and chlorine from mixing after they have been emitted at the electrodes.
Sodium metal increases in a special compartment from which it is taken out at intervals.
Advantages of Cell
The cell produces dry chlorine and 99.9 percent pure sodium. The procedure is performed at 600 ° C and it has the following advantages.
- (a) The metallic fog is not produced.
- (b) Liquid sodium can easily be collected at 600 ° C.
- (c) The product of the cell is not attacked by the products formed throughout the electrolysis.
Reactions in Cell
During the process, the following reactions occur:
NaCl à Na+ + Cl–
At cathode,
Na+ + e–à Na
At Anode,
Cl- à ½ Cl2 + 1e–
Properties of Sodium
- Sodium at standard temperature and pressure is a soft silvery metal that combines with oxygen in the air and forms grayish white sodium oxide unless immersed in oil or inert gas, which are the conditions it is typically kept in.
- Sodium metal can be easily cut with a knife and is an excellent conductor of electrical energy and heat because it has only one electron in its valence shell, leading to weak metal bonding and free electrons, which carry energy.
- In a flame test, sodium and its substances glow yellow.
- Twenty isotopes of sodium are known, but only 23Na is stable.
- Because of the high polarity of the C-Na bonds, they act as sources of carbanions (salts with organic anions). Some well-known derivatives include salt cyclopentadienide (NaC5H5) and trityl cyclopentadienide((C6H5) 3CNa).
Uses of Sodium
- Sodium is used as a heat exchanger in some nuclear reactors and as a reagent in the chemicals industry. However, sodium salts have more usage than the metal itself.
- The most typical compound of sodium is sodium chloride (common salt). It is added to food and utilized to de-ice roads in the winter season. It is likewise utilized as a feedstock for the chemical industry.
- Sodium carbonate (washing soda) is also a useful sodium salt. It is utilized as a water softener.
- Sodium is important to all living things, and humans have known this given prehistoric times. Our bodies contain about 100 grams, but we are continuously losing salt in different methods so we need to replace it. We can get all the sodium we need from our food, without including any extra. The average individual consumes about 10 grams of sodium a day, however, all we require has to do with 3 grams. Any extra sodium might contribute to hypertension. Sodium is essential for several functions of the body. For instance, it assists cells to transmit nerve signals and control water levels in tissues and blood.
- It is also utilized in improving the structure of certain alloys; soaps, filtration of molten metals, and sodium vapor lights.
- Sodium is essential in the manufacturing of natural substances and in making esters.
- Solid sodium carbonate is needed in making glass.
Multiple Choice Questions
- What is the chemical symbol for sodium?
- A) Ne
- B) Na
- C) Ni
- D) No
- Answer: B
- Where does sodium rank among the elements in terms of abundance in the Earth’s crust?
- A) 1st
- B) 3rd
- C) 6th
- D) 11th
- Answer: C
- How is sodium metal typically obtained?
- A) Extraction from ores
- B) Directly from nature as a free metal
- C) Electrolysis of dry molten sodium oxide
- D) Chemical reduction of sodium chloride
- Answer: C
- What is the primary compound of sodium found in nature?
- A) Sodium sulfate
- B) Sodium nitrate
- C) Sodium chloride
- D) Sodium carbonate
- Answer: C
- In the chemical preparation of salt by Downs Cell, what is the purpose of adding calcium chloride?
- A) To increase the melting point of sodium chloride
- B) To decrease the melting point of sodium chloride
- C) To enhance the conductivity of the electrolyte
- D) To produce dry chlorine gas
- Answer: B
- What is the anode material in Downs Cell during the electrolysis of sodium chloride?
- A) Copper
- B) Iron
- C) Graphite
- D) Sodium
- Answer: C
- What advantage does the Downs Cell process have in sodium production?
- A) Production of metallic fog
- B) Collection of liquid sodium at low temperatures
- C) Attack by products formed during electrolysis
- D) None of the above
- Answer: B
- What color does sodium and its compounds produce in a flame test?
- A) Red
- B) Blue
- C) Yellow
- D) Green
- Answer: C
- How many stable isotopes does sodium have?
- A) 15
- B) 20
- C) 23
- D) 30
- Answer: C
- What is the main use of sodium in nuclear reactors?
- A) Heat exchanger
- B) Electrical conductor
- C) Catalyst
- D) Fuel
- Answer: A
- Which sodium salt is commonly used for de-icing roads in winter?
- A) Sodium sulfate
- B) Sodium nitrate
- C) Sodium chloride
- D) Sodium carbonate
- Answer: C
- How much sodium does the average person consume daily, according to the passage?
- A) 1 gram
- B) 5 grams
- C) 10 grams
- D) 20 grams
- Answer: C
- What is the primary function of sodium in the human body?
- A) Enhancing muscle strength
- B) Transmitting nerve signals
- C) Supporting blood clotting
- D) Regulating body temperature
- Answer: B
- In the manufacturing of glass, what sodium compound is required?
- A) Sodium sulfate
- B) Sodium nitrate
- C) Sodium chloride
- D) Sodium carbonate
- Answer: D
Frequently Asked Questions (FAQs)
- Q: What is the chemical symbol and atomic number of sodium?
- A: The chemical symbol for sodium is Na, and its atomic number is 11.
- Q: Is sodium found in nature as a free metal?
- A: No, sodium is highly reactive and is never found as a free metal in nature. It is obtained from compounds.
- Q: How is sodium metal produced?
- A: Sodium metal is produced by electrolysis of dry molten sodium chloride.
- Q: What is the most common compound of sodium?
- A: Sodium chloride (NaCl) is the most common compound of sodium.
- Q: How is salt metal primarily produced, and why is calcium chloride added in the process?
- A: Most salt metal is produced by the electrolysis of fused sodium chloride. Calcium chloride is added to decrease the melting point of sodium chloride, allowing the process to occur at about 600°C.
- Q: What are the advantages of the Downs Cell process in sodium production?
- A: The advantages include the production of dry chlorine, obtaining 99.9 percent pure sodium, prevention of metallic fog, easy collection of liquid sodium at 600°C, and the product being resistant to attack by electrolysis by-products.
- Q: How many stable isotopes does sodium have?
- A: Sodium has one stable isotope, which is 23Na.
- Q: What are the primary uses of sodium?
- A: Sodium is used as a heat exchanger in nuclear reactors, a reagent in the chemicals industry, and in various sodium salts. Common salt (sodium chloride) is used in food, de-icing roads, and as a feedstock. Sodium carbonate is used as a water softener.
- Q: How much sodium does the average person consume daily, and why is it essential?
- A: The average person consumes about 10 grams of sodium a day, but the actual requirement is around 3 grams. Sodium is essential for transmitting nerve signals, controlling water levels, and has various functions in the body.
- Q: What are some notable derivatives of sodium?
- A: Sodium forms salts with organic anions, and well-known derivatives include salt cyclopentadienide (NaC5H5) and trityl cyclopentadienide ((C6H5)3CNa).
- Q: In what industries is sodium important?
- A: Sodium is important in the manufacturing of natural substances, making esters, improving the structure of certain alloys, soaps, filtration of molten metals, and sodium vapor lights.
- Q: Why is solid sodium carbonate needed in making glass?
- A: Solid sodium carbonate is required in making glass as it plays a crucial role in the glass-forming process.
Summary
In this comprehensive tutorial on Sodium and its preparation, we explored various aspects of this essential chemical element.
- Introduction to Sodium:
- Sodium (Na), with atomic number 11, is a highly reactive metal in group 1 of the periodic table.
- It is not found in nature as a free metal but is obtained from compounds.
- Chemical Preparation of Sodium:
- Sodium metal is produced by the electrolysis of dry molten sodium chloride.
- Downs Cell is commonly used, where calcium chloride is added to reduce the melting point.
- Advantages of Downs Cell:
- The cell produces dry chlorine and 99.9% pure sodium.
- Conducted at 600°C, it prevents the production of metallic fog and allows easy collection of liquid sodium.
- Properties of Sodium:
- At standard temperature and pressure, sodium is a soft silvery metal that reacts with oxygen to form sodium oxide.
- It can be easily cut with a knife, conducts electricity and heat well, and exhibits a yellow glow in a flame test.
- There are twenty isotopes of sodium, with 23Na being stable.
- Uses of Sodium:
- Sodium is used as a heat exchanger in nuclear reactors and as a reagent in the chemicals industry.
- Sodium salts, particularly sodium chloride, have diverse applications, including food seasoning and de-icing roads.
- Sodium carbonate serves as a water softener.
- Significance in Living Organisms:
- Sodium is vital for transmitting nerve signals and regulating water levels in tissues and blood.
- While the average person consumes about 10 grams of sodium daily, the actual requirement is around 3 grams.
- Industrial Applications:
- Sodium is used in improving the structure of alloys, manufacturing soaps, filtration of molten metals, and sodium vapor lights.
- It is essential in the production of natural substances and esters.
- Solid sodium carbonate is a key component in making glass.
This tutorial provides a comprehensive understanding of sodium, its preparation methods, properties, and the wide range of applications that make it an indispensable element in various industries and biological processes.

