Discovery of Sub-atomic particles
The journey into the discovery of subatomic particles begins with Dalton’s perspective, envisioning atoms as indivisible and dense spheres, where atoms of the same element share similarities and combine to form compounds.
Building on Dalton’s atomic theory, scientists delved into experiments during the late 1800s and early 1900s, unraveling new layers of understanding. In 1886, Goldstein identified positively charged particles, termed protons, while J.J. Thomson, in 1897, revealed the existence of negatively charged particles—electrons—within the atom.
This marked the establishment of electrons and protons as the fundamental constituents of matter.
Thomson’s “plum pudding” theory conceptualized atoms as robust structures positively charged, housing minuscule negatively charged particles akin to plums in a pudding.
- 1) Cathode rays and Discovery of Electron
- 2) Characteristic of Cathode rays
- 3) Discovery of Proton
- 4) Properties
- 5) Discovery of Neutron
- 6) Frequently Asked Questions (FAQs) – Discovery of Sub-atomic Particles
- 7) Multiple-Choice Questions (MCQs) – Discovery of Sub-atomic Particles
- 8) Wrapping up
- 9) You may also like to learn:
Cathode rays and Discovery of Electron
In 1895 Sir William Crooks performed experiments by passing an electrical current through gases in a discharge tube at extremely low pressure.
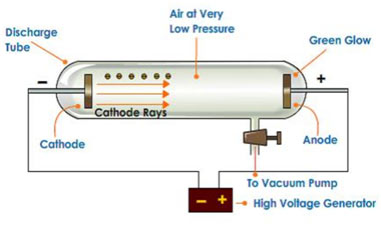
He took a glass tube fitted with two metallic electrodes, which were linked to a high voltage battery. The pressure inside the tube was kept 10-4 atm. When high voltage current was gone through the gas, glossy rays were given off from the cathode which traveled towards the anode. These rays were offered the name of “cathode rays” as these were originated from the cathode.
Characteristic of Cathode rays
The cathode rays were studied in detail and their properties were determined, which are listed below:
- These rays travel in straight lines perpendicular to the cathode surface.
- They can cast a sharp shadow of an opaque object if positioned in their course.
- They are deflected towards the positive plate in an electrical field showing that they are negatively charged.
- They raise the temperature level of the body on which they fall.
- Thomson discovered their charge/mass (e/ m) ratio.
- Light is produced when these rays hit the walls of the discharge tube.
- It was found that the very same kind of rays were discharged no matter which gas and which cathode was used in the discharge tube.
All these properties suggested that the nature of cathode rays is independent of the nature of the gas present in the discharge tube or product of the cathode. The fact that they cast the shadow of an opaque item suggested that these are not rays however they are quick moving material particles.
They were offered the name electrons. Given that all the materials produce exact the same kind of particles, it implies all the materials contain electrons. As we know materials are made up of atoms, thus the electrons are fundamental particles of atoms.
Discovery of Proton
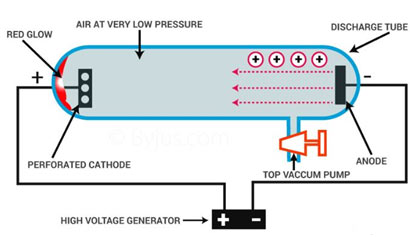
In 1886 Goldstein observed that in addition to cathode rays, other rays were also present in the discharge tube. These rays were traveling in the opposite direction to cathode rays. He used a discharge tube having a perforated cathode. He discovered that these rays traveled through holes present in the cathode and produced a radiance on the walls of the discharge tube. He called these rays as “canal rays”.
Properties
The properties of canal rays were as following:
- These rays travel in straight lines in a direction opposite to the cathode rays.
- Their deflection in the electric and magnetic fields proved that these are positively charged.
- The nature of canal rays depends upon the nature of gas, present in the discharge tube.
- These rays do not stem from the anode. In fact, these rays are produced when the cathode rays or electrons collide with the residual gas molecules present in the discharge tube and ionize them as follows:
![]()
The mass of these particles was found equal to that of a proton or easy multiple of it. The mass of a proton is 1840 times more than that of an electron.
Hence, these rays are made up of positively charged particles. The mass and charge of these particles depend upon the nature of the gas in the discharge tube. Hence, various gases produce various kinds of positively charged rays having particles of different masses. Remembering that positive particles produced by gas will be of the exact same type i.e. positive rays produced by the lightest gas hydrogen include protons.
Discovery of Neutron
Rutherford observed that the atomic mass of the element could not be explained on the basis of the masses of electron and proton only. He forecasted in 1920 that some neutral particles having a mass equal to that of protons need to exist in an atom. Thus, scientists remained in search of such a neutral particle.
Eventually, in 1932 Chadwick discovered neutron when he bombarded alpha particles on a beryllium target. He observed that highly penetrating radiations were produced. These radiations were called the neutron.
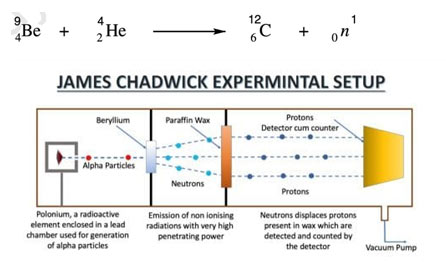
Properties of the neutron are as follows:
- Neutrons carry no charge i.e. they are neutral.
- They are highly penetrating.
- Mass of these particles was almost equal to the mass of a proton.
Frequently Asked Questions (FAQs) – Discovery of Sub-atomic Particles
- What did Dalton propose about the atom, and how did it influence early atomic theory?
- Dalton proposed that an atom is an indivisible and dense sphere, and atoms of the same element are alike. This idea influenced early atomic theory and led to experiments to explore the structure of atoms.
- Who discovered the sub-atomic particles, electrons, and protons, and when did these discoveries take place?
- J.J. Thomson discovered electrons in 1897, and Goldstein found protons in 1886.
- What is the “plum pudding” model, and who proposed it based on the discoveries of electrons and protons?
- The “plum pudding” model was proposed by J.J. Thomson. It suggests that atoms are solid structures of positive charge with tiny, negatively charged particles (electrons) embedded inside, resembling plums in a pudding.
- How were cathode rays used to discover electrons?
- Sir William Crookes performed experiments with cathode rays in a discharge tube, leading to the discovery of electrons. These rays were found to be negatively charged particles with specific properties.
- What are the characteristics of cathode rays?
- Cathode rays travel in straight lines perpendicular to the cathode surface, cast sharp shadows, are deflected towards the positive plate in an electrical field, raise the temperature of objects they hit, and have a charge/mass (e/m) ratio.
- Why were cathode rays named electrons, and what do they reveal about the nature of matter?
- Cathode rays were named electrons because they are fundamental particles present in all materials. This implies that electrons are fundamental particles of atoms, contributing to the understanding of matter.
- Who discovered the proton, and what are the properties of canal rays?
- Goldstein discovered the proton in 1886. Canal rays travel in straight lines opposite to cathode rays, are positively charged, and their nature depends on the gas present in the discharge tube.
- How did Rutherford’s observations lead to the prediction and discovery of neutrons?
- Rutherford’s observations on atomic mass led to the prediction of neutral particles with masses similar to protons. Neutrons were discovered by Chadwick in 1932 when bombarding alpha particles on a beryllium target.
- What are the properties of neutrons?
- Neutrons carry no charge (neutral), are highly penetrating, and have a mass almost equal to that of a proton.
- Why did scientists believe that neutral particles like neutrons must exist in an atom?
- Scientists believed that neutral particles like neutrons must exist to explain the atomic mass of elements, as the masses of electrons and protons alone couldn’t account for it.
Q11: Difference between neutron and proton.
| Proton | Neutron |
| Positive subatomic particle | Neutral subatomic particle |
| Mass of proton is 1.67 x 10 – 27 kg | Mass of neutron is 1.67493 × 10−27 kg |
Q12: What was the pressure inside the discharge tube for cathode rays?
Ans: For cathode rays, the pressure inside the discharge tube was kept 10-4 atm.
Q13: What is charge to mass ratio of cathode rays?
Ans: The charge to mass ratio of cathode rays is 1. 76×108C/g and is independent of nature of gas in the discharge tube.
Q14: What are canal rays?
Ans: Goldstein observed that in addition to cathode rays, other rays were also present in the discharge tube. These travel in opposite direction. He named them canal rays.
Multiple-Choice Questions (MCQs) – Discovery of Sub-atomic Particles
- What did Dalton propose about atoms in his atomic theory?
- A. Atoms are indivisible and dense spheres.
- B. Atoms are made up of electrons, protons, and neutrons.
- C. Atoms are continuously divisible into smaller particles.
- D. Atoms are invisible and intangible.
Answer: A. Atoms are indivisible and dense spheres.
- Who discovered positively charged particles called protons?
- A. J.J. Thomson
- B. Ernest Rutherford
- C. Sir William Crookes
- D. Goldstein
Answer: D. Goldstein
- When were electrons discovered, and who made this discovery?
- A. 1897, Goldstein
- B. 1886, J.J. Thomson
- C. 1895, Sir William Crookes
- D. 1932, Chadwick
Answer: C. 1897, J.J. Thomson
- What theory did J.J. Thomson propose based on his observations of electrons?
- A. Plum Pudding Theory
- B. Quantum Theory
- C. Nuclear Theory
- D. Wave-Particle Duality Theory
Answer: A. Plum Pudding Theory
- How were cathode rays produced in Sir William Crookes’ experiments?
- A. By passing light through a vacuum tube
- B. By passing an electric current through gases at low pressure
- C. By bombarding alpha particles on a target
- D. By heating a cathode in the presence of a magnetic field
Answer: B. By passing an electric current through gases at low pressure
- What property of cathode rays indicated their negative charge?
- A. Straight-line travel
- B. Deflection towards the anode
- C. Production of light when hitting walls
- D. Raising the temperature of objects
Answer: B. Deflection towards the anode
- Why were cathode rays named electrons?
- A. They were discovered by Crookes.
- B. They were positively charged.
- C. They were found to be fundamental particles in all materials.
- D. They cast shadows of opaque objects.
Answer: C. They were found to be fundamental particles in all materials.
- Who discovered the proton, and how were canal rays related to its discovery?
- A. J.J. Thomson, produced in discharge tube
- B. Goldstein, traveling opposite to cathode rays
- C. Sir William Crookes, deflected in electric fields
- D. Ernest Rutherford, bombarding alpha particles
Answer: B. Goldstein, traveling opposite to cathode rays
- What is the nature of canal rays?
- A. Negatively charged
- B. Neutral
- C. Positively charged
- D. Both A and C
Answer: C. Positively charged
- Why did Rutherford predict the existence of neutrons?
- A. To explain the nature of cathode rays
- B. To account for the atomic mass of elements
- C. To study the deflection of electrons
- D. To understand the properties of protons
Answer: B. To account for the atomic mass of elements
- Who discovered neutrons, and how were they produced?
- A. J.J. Thomson, cathode rays
- B. Goldstein, canal rays
- C. Rutherford, alpha particles on beryllium
- D. Chadwick, bombarding alpha particles
Answer: D. Chadwick, bombarding alpha particles
- What is the charge of neutrons?
- A. Positive
- B. Negative
- C. Neutral
- D. Variable
Answer: C. Neutral
- What property of neutrons makes them highly penetrating?
- A. High speed
- B. Negative charge
- C. Neutral charge
- D. High mass
Answer: C. Neutral charge
- What is the mass relationship between protons and electrons?
- A. Mass of protons is less than electrons
- B. Mass of protons is equal to electrons
- C. Mass of protons is greater than electrons
- D. Mass of protons is inversely proportional to electrons
Answer: C. Mass of protons is greater than electrons
- Which particle is fundamental to the structure of all materials, according to the discoveries discussed?
- A. Electrons
- B. Protons
- C. Neutrons
- D. Cathode rays
Answer: A. Electrons
Wrapping up
The journey into the subatomic realm began with Dalton’s atomic theory, which considered atoms as indivisible and dense spheres. However, in the late 1800s and early 1900s, groundbreaking experiments led to the discovery of new subatomic particles, challenging Dalton’s atomic indivisibility.
Goldstein’s work in 1886 revealed the existence of positively charged particles known as protons, while J.J. Thomson, in 1897, identified negatively charged electrons within the atom. Thomson proposed the “plum pudding” model, envisioning atoms as structures of positive charge with embedded electrons resembling plums in a pudding.
Sir William Crookes, in 1895, conducted experiments with cathode rays, revealing properties such as straight-line travel, shadow casting, and negative charge deflection in electrical fields. Thomson’s discovery of the charge-to-mass ratio and the consistent nature of cathode rays across different gases led to the identification of these fast-moving particles as electrons, fundamental to all materials.
Goldstein’s observations of rays traveling opposite to cathode rays, later termed canal rays, unveiled the discovery of protons. These positively charged particles were found to travel in straight lines and were influenced by electric and magnetic fields. Their mass, substantially greater than electrons, varied with the nature of the gas in the discharge tube.
Rutherford’s studies prompted the prediction of neutral particles, eventually identified as neutrons by Chadwick in 1932. Neutrons, lacking a charge, displayed high penetration capabilities, and their mass closely resembled that of protons.
In conclusion, the discovery of electrons, protons, and neutrons transformed the understanding of atomic structure, paving the way for advancements in atomic theory and contributing to the foundation of modern physics.