Nuclear Reactions
The structure of the nucleus undergoes changes due to the bombardment of fast-moving particles such as neutrons, α- particles, protons, etc. on a targeted nucleus, such reactions are called nuclear reactions.
![]()
- 1) Conditions for Nuclear Reactions
- 2) Nuclear Fission Reactions
- 3) Discovery
- 4) Fission Chain Reactions
- 5) Controlled Chain Reaction
- 6) Uncontrolled Chain Reaction
- 7) Amount of energy in Fission reaction
- 8) MCQs About Fission Reaction
- 9) Summary Understanding Nuclear Fission Reactions
- 10) You may also like to learn:
Conditions for Nuclear Reactions
Following conditions are necessary for the nuclear reactions to take place.
Conservation of mass: The number of protons and neutrons in the nucleus remains the same because these are not produced neither destroyed.
![]()
In this reaction,
Number of protons: 7+2= 8+1
Number of neutrons: 7+2= 9+0
Conservation of Energy: The net energy including the rest of mass-energy remains constant before and after the nuclear reaction. In the above reaction;

Difference in mass= 18. 0069 – 18. 0057 = 0.0012
This energy should be supplied to reactants to start this reaction.
Nuclear Fission Reactions
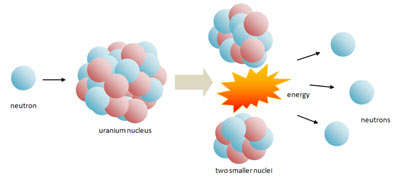
The reaction in which heavy nucleus such as Uranium 23592 U is split down into two nuclei of nearly equal size as a result of bombardment of particles along with the release of energy. Such a reaction is called nuclear fission.
Discovery
Two German scientists named Otto Hahn and Fritz Strassmann, in 1939 made a very important discovery. They bombarded a sample of Uranium with slow neutrons. A nuclear reaction was initiated.
As a result of the reaction, barium and krypton were formed, 3 neutrons were released along with the emission of 200 Me V energy.

Fission reaction is possible for Uranium and other heavy elements. Uranium and Plutonium are the most commonly used elements for fission purposes as they are very easy to control and initiate. The energy produced by the fission reaction is used to heat water, which converts it into steam. The steam is used to run turbines and generators for the production of electricity.
Fission Chain Reactions
A chain reaction refers to a process in which by efficiently using emitted (2 or 3) neutrons can further produce more Uranium atoms such that, the reaction can continuously maintain itself. Following are the two types of fission chain reactions.
Controlled Chain Reaction
The conditions are produced in which out of all neutrons produced, only one can further initiate a fission reaction. The other neutrons are absorbed by some medium or escape out of reaction. The fission reaction with the constant initial speed in a controlled environment is called a controlled chain reaction.
Critical mass: The minimum mass of Uranium capable of a sustained fission reaction is called the critical mass.
Uncontrolled Chain Reaction
The three neutrons emitted along with the barium and krypton, initiate further three fission reactions. As a result, 23592 U and 9 neutrons are produced. These 9 neutrons will initiate additional fission.

In this way, multiple fission reactions will occur and the chain goes on. This process is called an uncontrolled chain reaction. Multiple fission can cause an atomic explosion and a huge amount of energy is released.
Amount of energy in Fission reaction
Nuclear reactions liberate a large amount of energy compared to chemical reactions. One fission reaction results in the emission of about 200 MeV of energy, or about 3.2 x 10-11 watt-seconds. Therefore, 3.1 x 1010 fissions per second produce1 W of thermal power.
The fission of 1 g of uranium or plutonium per day liberates about 1 MW. This is the energy equivalent of 3 tons of coal or about 600 gallons of fuel oil each day, which when burned produces around 1/4 tons of carbon dioxide.
Atomic power plants make their own fuel, given that they produce 239Pu from 238U. With the overall around the world installed nuclear capacity of 3.4 x 105 MWe (megawatt electrical), one can estimate that more than 100 tons of 239Pu are produced each year in reactors whose primary energy source is the fission of 235U. This 239Pu can be reprocessed from used fuel rods and utilized to power other reactors.
MCQs About Fission Reaction
- What are nuclear reactions?
- A. Chemical reactions involving electrons
- B. Changes in the nucleus due to particle bombardment
- C. Reactions occurring only in nuclear power plants
- D. Reactions involving only protons
- Answer: B. Changes in the nucleus due to particle bombardment
- Which condition must be met for nuclear reactions to occur?
- A. Conservation of electrons
- B. Conservation of mass and energy
- C. Increase in mass and energy
- D. Decrease in mass and energy
- Answer: B. Conservation of mass and energy
- What is nuclear fission?
- A. Combining two light nuclei to form a heavier one
- B. Splitting a heavy nucleus into two nearly equal-sized nuclei
- C. Converting mass into energy in a nuclear reactor
- D. Absorbing neutrons in a nuclear chain reaction
- Answer: B. Splitting a heavy nucleus into two nearly equal-sized nuclei
- Who discovered nuclear fission?
- A. Marie Curie
- B. Albert Einstein
- C. Otto Hahn and Fritz Strassmann
- D. Enrico Fermi
- Answer: C. Otto Hahn and Fritz Strassmann
- What are the most commonly used elements for nuclear fission?
- What is the minimum mass of Uranium capable of sustaining a fission reaction called?
- A. Reaction mass
- B. Fission mass
- C. Critical mass
- D. Controlled mass
- Answer: C. Critical mass
- What type of chain reaction involves only one neutron from each fission reaction causing another fission?
- A. Uncontrolled chain reaction
- B. Controlled chain reaction
- C. Limited chain reaction
- D. Nuclear chain reaction
- Answer: B. Controlled chain reaction
- What is the condition for an uncontrolled chain reaction?
- A. Only one neutron initiates further fission reactions
- B. Neutrons are absorbed by a medium or escape the reaction
- C. Multiple neutrons from each fission reaction cause further fissions
- D. Only one neutron is released in each fission reaction
- Answer: C. Multiple neutrons from each fission reaction cause further fissions
- How much energy does one fission reaction release?
- A. 20 MeV
- B. 100 MeV
- C. 200 MeV
- D. 500 MeV
- Answer: C. 200 MeV
- How much thermal power is produced by 3.1 x 10^10 fissions per second?
- A. 0.32 W
- B. 1 W
- C. 3.1 MW
- D. 10 MW
- Answer: B. 1 W
- How much energy is released by the fission of 1 g of uranium or plutonium per day?
- A. 1 kWh
- B. 1 MW
- C. 1 GW
- D. 1 TW
- Answer: B. 1 MW
- What is produced from the fission of 238U in nuclear reactors?
- A. Helium
- B. Carbon dioxide
- C. Plutonium (239Pu)
- D. Oxygen
- Answer: C. Plutonium (239Pu)
- What is the overall installed nuclear capacity worldwide?
- A. 1 MW
- B. 10 MW
- C. 100 MW
- D. 3.4 x 10^5 MWe
- Answer: D. 3.4 x 10^5 MWe
- How much plutonium (239Pu) is estimated to be produced each year in reactors?
- A. Less than 10 tons
- B. About 50 tons
- C. More than 100 tons
- D. Exactly 239 tons
- Answer: C. More than 100 tons
- What can be reprocessed from used fuel rods and used to power other reactors?
- A. Uranium
- B. Plutonium (239Pu)
- C. Neutrons
- D. Electrons
- Answer: B. Plutonium (239Pu)
- How much energy does the fission of 1 g of uranium or plutonium per day liberate?
- A. Equivalent to 3 tons of coal
- B. Equivalent to 1 kWh
- C. Equivalent to 10 liters of fuel oil
- D. Equivalent to 1 TW
- Answer: A. Equivalent to 3 tons of coal
- How much carbon dioxide is produced daily by the burning of fuel oil equivalent to the energy from fission?
- A. Less than 1 ton
- B. About 1 ton
- C. More than 1 ton
- D. Exactly 3 tons
- Answer: D. Exactly 3 tons
- What type of reaction liberates a large amount of energy compared to chemical reactions?
- A. Nuclear fusion
- B. Nuclear fission
- C. Combustion
- D. Oxidation
- Answer: B. Nuclear fission
- What does one fission reaction result in the emission of?
- A. 200 keV
- B. 200 MeV
- C. 200 GeV
- D. 200 TeV
- Answer: B. 200 MeV
Summary Understanding Nuclear Fission Reactions
Introduction to Nuclear Reactions
Nuclear reactions involve changes in the nucleus due to the bombardment of particles such as neutrons, alpha particles, and protons. These reactions are fundamental to understanding nuclear processes.
Conditions for Nuclear Reactions
Certain conditions, including the conservation of mass and energy, are necessary for nuclear reactions to occur. Understanding these conditions is crucial for harnessing nuclear energy effectively.
Exploring Nuclear Fission Reactions
Nuclear fission reactions entail the splitting of heavy nuclei, such as Uranium-235, into smaller nuclei, releasing substantial energy. This section delves into the intricacies of nuclear fission.
Discovering Nuclear Fission
The discovery of nuclear fission by Otto Hahn and Fritz Strassmann in 1939 marked a significant milestone in scientific history. This discovery laid the foundation for further exploration and application of nuclear energy.
Understanding Fission Chain Reactions
Fission chain reactions play a pivotal role in sustaining or amplifying the fission process. This section examines the mechanisms behind controlled and uncontrolled chain reactions.
Controlled Chain Reaction: Managing Nuclear Energy
Controlled chain reactions are carefully regulated to maintain a constant speed, ensuring safe and efficient energy production. Understanding the principles of controlled chain reactions is essential for nuclear power generation.
Uncontrolled Chain Reaction: Exploring Nuclear Hazards
Uncontrolled chain reactions, if left unchecked, can lead to catastrophic events such as atomic explosions. This section highlights the risks associated with uncontrolled fission reactions.
Energy Release in Fission Reactions
Nuclear fission reactions release a substantial amount of energy compared to chemical reactions. This section explores the energy dynamics involved in nuclear fission and its implications for power generation.
Harnessing Nuclear Power: Practical Applications
Nuclear power plants utilize the energy generated by fission reactions to produce electricity. This section discusses the practical applications of nuclear energy and its role in meeting global energy demands.
Conclusion: The Promise of Nuclear Energy
In conclusion, nuclear fission reactions hold immense potential as a source of clean and sustainable energy. Understanding the principles and applications of nuclear fission is crucial for realizing this promise and addressing future energy challenges.