Spectrum
A spectrum is specified as the characteristic wavelengths of electromagnetic radiation (or a part thereof) that is produced, emitted or absorbed by an object substance, atom, or molecule.
Example
Examples of a spectrum consist of the rainbow, the emission colours from the Sun, and the infrared absorption wavelengths from a molecule.
Explanation of Spectrum
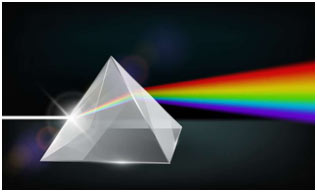
When radiation of light is passed through a prism, the radiation goes through refraction or bending. The level of bending relies on the wavelength of the photons. Radiation of longer wavelength is bent to a smaller degree than the radiation of a shorter wavelength. Ordinary, white light includes radiation of all wavelengths, therefore after travelling through the prism, white light is broken up into radiations of different wavelengths.
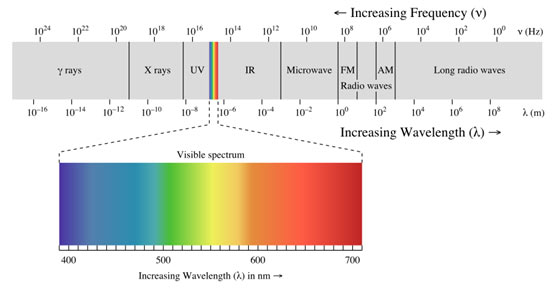
The colours of the visible spectrum are violet, indigo, blue, green, orange, yellow and red and their wavelengths vary from 400 nm to 750 nm. In addition to the visible region of the spectrum, there are 7 other regions.
Ultraviolet, X-rays, γ-rays and cosmic rays are towards the lower wavelength end of the spectrum and they have the photons with higher energies. On the other side of the visible region, there lie infrared, microwave and radio-frequency regions. Hence, a visual display or dispersion of the components of white light, when it is passed through a prism is called a spectrum.
Types of Spectrum
Spectrum is of two types:
- (i) Continuous spectrum
- (ii) Line spectrum
Continuous spectrum
In this kind of spectrum, the boundary line in between the colours cannot be marked. The colours diffuse into each other. One colour merges into another without any dark space. The very best example of a continuous spectrum is rainbow. It is obtained from the light released by the sun or incandescent (electrical light) solids. It is the characteristic of matter in bulk.
Atomic or Line Spectrum
When an element or its compound is volatilized on a flame and the light released is seen through a spectrometer. We see unique lines separated by dark areas. This kind of spectrum is called a line spectrum or atomic spectrum. This is characteristic of an atom.
The variety of lines and the range in between them depend upon the element volatilized. For example, the line spectrum of sodium consists of 2 yellow coloured lines separated by a definite distance. Similarly, the spectrum of hydrogen consists of a number of lines of different colours having different distances from each other. It has actually also been observed that distances between the lines for the hydrogen spectrum decline with the decline in wavelength and the spectrum end up being continuous after a certain value of wavelength.
Atomic spectrum can likewise be observed when elements in the gaseous state are heated at high temperature or subjected to an electric discharge.
There are two ways in which an atomic spectrum can be seen.
- (i)Atomic emission spectrum
(ii) Atomic absorption spectrum
Atomic Emission Spectrum
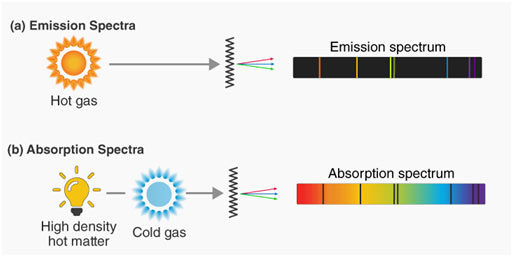
When solids are volatilized or elements in their gaseous states are heated to high temperature or subjected to an electrical discharge, radiation of specific wavelengths are emitted. The spectrum of this radiation contained bright lines against a dark background. This is called an atomic emission spectrum.
Atomic Absorption Spectrum
When a beam of white light is passed through a gaseous sample of an element, the element takes in certain wavelengths while the rest of wavelengths travel through it. The spectrum of this radiation is called an atomic absorption spectrum. The wavelengths of the radiation that have actually been absorbed by the element look like dark lines and the background are bright.
It is interesting to keep in mind that the positions or the wavelengths of lines appearing in both emission and absorption spectra are precisely the same. In emission spectrum, these lines appear bright due to the fact that the matching wavelengths are being given off by the element, whereas they appear dark in absorption spectrum because the wavelengths are being absorbed by the element.
Hydrogen Spectrum
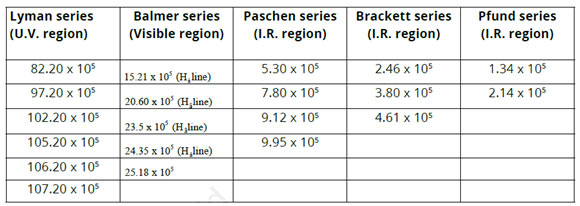
Hydrogen-spectrum is an important example of the atomic spectrum. Hydrogen is filled in a discharge tube at a very low pressure a bluish light is given off from the discharge tube. This light when viewed through a spectrometer shows numerous separated sharp lines. These are called spectral lines. The wavelengths of these lines depend on the visible, ultraviolet and infrared areas. These spectral lines can be classified into five groups called spectral series. These series are named after their innovators as revealed listed below.
- (i) Lyman series (U.V region)
- (ii) Balmer series (visible region)
- (iii) Paschen series (LR region)
- (iv) Brackett series (I.R region)
- (v) Pfund series (I.R region)

The very first 4 series were discovered before Bohr’s atomic design (1913). It is seen that as we proceed from Lyman series to Pfund series, the wave numbers (m-1) of spectral lines reduce. The lines of Balmer series have actually been given specific names as H α, H ß … …, etc.
MCQs about Spectrum
- What is a spectrum?
- a. A type of chemical reaction
- b. Characteristic wavelengths of electromagnetic radiation
- c. A type of solution in chemistry
- d. A component of atoms
Answer: b. Characteristic wavelengths of electromagnetic radiation
- When radiation of light is passed through a prism, what happens?
- a. Reflection
- b. Absorption
- c. Refraction
- d. Diffusion
Answer: c. Refraction
- Which colors are part of the visible spectrum?
- a. Red, Green, Blue
- b. Yellow, Orange, Violet
- c. Violet, Indigo, Blue, Green, Orange, Yellow, Red
- d. Black and White
Answer: c. Violet, Indigo, Blue, Green, Orange, Yellow, Red
- What are the regions beyond the visible spectrum?
- a. Ultraviolet, X-rays, γ-rays, and cosmic rays
- b. Infrared, Microwave, and Radio-frequency
- c. Both a and b
- d. None of the above
Answer: c. Both a and b
- What is a continuous spectrum characterized by?
- a. Clearly defined boundaries between colors
- b. Colors merging into each other without dark spaces
- c. Absence of colors
- d. Complete darkness
Answer: b. Colors merging into each other without dark spaces
- Which spectrum is obtained from the light released by the sun or incandescent solids?
- a. Atomic spectrum
- b. Line spectrum
- c. Continuous spectrum
- d. Emission spectrum
Answer: c. Continuous spectrum
- What is the characteristic of an atomic spectrum?
- a. Clear boundaries between colors
- b. Merging of colors
- c. Unique lines separated by dark areas
- d. Complete darkness
Answer: c. Unique lines separated by dark areas
- What does the number of lines in an atomic spectrum depend on?
- a. Temperature
- b. Volume
- c. Element volatilized
- d. Pressure
Answer: c. Element volatilized
- What is an Atomic Emission Spectrum characterized by?
- a. Dark lines against a bright background
- b. Bright lines against a dark background
- c. Merging of colors
- d. Clear boundaries between colors
Answer: b. Bright lines against a dark background
- What is an Atomic Absorption Spectrum characterized by?
- a. Bright lines against a dark background
- b. Merging of colors
- c. Dark lines against a bright background
- d. Clear boundaries between colors
Answer: c. Dark lines against a bright background
- What is the hydrogen spectrum an example of?
- a. Line spectrum
- b. Continuous spectrum
- c. Emission spectrum
- d. All of the above
Answer: a. Line spectrum
- What is the hydrogen spectral series named after?
- a. Colors
- b. Wavelengths
- c. Innovators
- d. Temperature
Answer: c. Innovators
- Which series of the hydrogen spectrum is in the infrared region?
- a. Lyman series
- b. Balmer series
- c. Paschen series
- d. Brackett series
Answer: c. Paschen series
- In the hydrogen spectrum, which series is in the visible region?
- a. Lyman series
- b. Balmer series
- c. Paschen series
- d. Brackett series
Answer: b. Balmer series
- What happens to the wave numbers of spectral lines as we proceed from Lyman series to Pfund series?
- a. Increase
- b. Remain constant
- c. Decrease
- d. Fluctuate
Answer: c. Decrease
- What are the lines of the Balmer series in the hydrogen spectrum named as?
- a. A, B, C, …
- b. X, Y, Z, …
- c. H α, H ß, …
- d. 1, 2, 3, …
Answer: c. H α, H ß, …
- What does the spectrum look like when white light is passed through a prism?
- a. Continuous spectrum
- b. Line spectrum
- c. Emission spectrum
- d. Absorption spectrum
Answer: a. Continuous spectrum
- Which spectrum is characteristic of an atom?
- a. Continuous spectrum
- b. Emission spectrum
- c. Line spectrum
- d. Absorption spectrum
Answer: c. Line spectrum
- What determines the bending of radiation in a spectrum?
- a. Temperature
- b. Wavelength
- c. Pressure
- d. Volume
Answer: b. Wavelength
- What is the hydrogen spectrum filled in for observation?
- a. Flask
- b. Beaker
- c. Test tube
- d. Discharge tube
Answer: d. Discharge tube
- Which region of the spectrum has photons with higher energies?
- a. Visible region
- b. Infrared region
- c. Ultraviolet, X-rays, γ-rays, and cosmic rays
- d. Radio-frequency region
Answer: c. Ultraviolet, X-rays, γ-rays, and cosmic rays
- In the electromagnetic spectrum, what is beyond the visible spectrum?
- a. Infrared, Microwave, and Radio-frequency
- b. Visible light only
- c. Ultraviolet, X-rays, γ-rays, and cosmic rays
- d. Both a and c
Answer: d. Both a and c
- What is the purpose of a spectrometer?
- a. To heat substances
- b. To absorb radiation
- c. To disperse the components of white light
- d. To generate electromagnetic radiation
Answer: c. To disperse the components of white light
Frequently Asked Questions (FAQs) – Spectrum
- What is a spectrum?
- A spectrum refers to the characteristic wavelengths of electromagnetic radiation produced, emitted, or absorbed by an object, substance, atom, or molecule.
- Can you provide examples of a spectrum?
- Examples of a spectrum include the rainbow, emission colors from the Sun, and the infrared absorption wavelengths from a molecule.
- How is a spectrum formed when light passes through a prism?
- When light passes through a prism, it undergoes refraction or bending. The extent of bending depends on the wavelength of the photons, leading to the separation of white light into radiations of different wavelengths.
- What are the colors of the visible spectrum, and what are their wavelengths?
- The colors of the visible spectrum are violet, indigo, blue, green, orange, yellow, and red. Their wavelengths range from 400 nm to 750 nm.
- What regions exist beyond the visible spectrum?
- Beyond the visible spectrum, there are regions such as ultraviolet, X-rays, γ-rays (higher energy end), and infrared, microwave, and radio-frequency (lower energy end).
- What are the types of spectra?
- There are two types of spectra: continuous spectrum and line spectrum.
- Can you provide an example of a continuous spectrum?
- The rainbow is a classic example of a continuous spectrum. It is obtained from light emitted by the sun or incandescent solids.
- What is an atomic or line spectrum?
- An atomic or line spectrum is formed when an element or its compound is volatilized, and the emitted light is observed through a spectrometer, revealing unique lines separated by dark areas.
- How does an atomic emission spectrum differ from an atomic absorption spectrum?
- An atomic emission spectrum contains bright lines against a dark background, while an atomic absorption spectrum shows dark lines against a bright background, representing absorbed wavelengths.
- What is the significance of the hydrogen spectrum?
- The hydrogen spectrum is crucial as it showcases the atomic spectrum. When hydrogen is filled in a discharge tube at low pressure, it emits bluish light with separated sharp lines called spectral lines.
- How is the hydrogen spectrum classified, and what are the series names?
- The hydrogen spectrum is classified into five groups known as spectral series, including Lyman series (U.V region), Balmer series (visible region), Paschen series (LR region), Brackett series (I.R region), and Pfund series (I.R region).
- What happens to the wave numbers of spectral lines as we move from Lyman to Pfund series?
- The wave numbers (m-1) of spectral lines decrease as we proceed from Lyman series to Pfund series.
- Are the positions or wavelengths of lines the same in emission and absorption spectra?
- Yes, the positions or wavelengths of lines in both emission and absorption spectra are exactly the same. In emission spectra, lines appear bright, while in absorption spectra, they appear dark.
- How is an atomic spectrum observed?
- An atomic spectrum can be observed in two ways: atomic emission spectrum, where specific wavelengths are emitted when solids or gaseous elements are heated, and atomic absorption spectrum, where certain wavelengths are absorbed when white light passes through a gaseous sample of an element.
Summary – Spectrum Tutorial
The spectrum, defined as characteristic wavelengths of electromagnetic radiation produced, emitted, or absorbed by objects, substances, atoms, or molecules, is a diverse and fascinating field. The tutorial covers the following key points:
- Definition and Examples of Spectrum:
- A spectrum encompasses characteristic wavelengths of electromagnetic radiation. Examples include the rainbow, emission colors from the Sun, and infrared absorption from molecules.
- Explanation of Spectrum:
- When light undergoes refraction through a prism, its wavelength determines the degree of bending. White light, with all wavelengths, is dispersed into various radiations, including violet, indigo, blue, green, orange, yellow, and red.
- Types of Spectrum:
- Spectrum is categorized into two types: continuous spectrum and line spectrum.
- Continuous Spectrum:
- Characterized by colors merging seamlessly without distinct boundaries, the continuous spectrum is exemplified by the rainbow, derived from sunlight or incandescent solids.
- Atomic or Line Spectrum:
- Resulting from the volatilization of elements, an atomic or line spectrum exhibits unique lines. For instance, sodium’s spectrum includes two yellow lines, while hydrogen’s spectrum features multiple lines with varying distances.
- Atomic Emission and Absorption Spectrum:
- Atomic emission spectrum arises when solids or gaseous elements emit specific wavelengths, forming bright lines. In contrast, an atomic absorption spectrum occurs when an element absorbs certain wavelengths from white light, resulting in dark lines.
- Hydrogen Spectrum:
- The hydrogen spectrum, a crucial atomic example, is observed when hydrogen is excited in a discharge tube. Spectral lines are categorized into series such as Lyman, Balmer, Paschen, Brackett, and Pfund, each associated with specific regions.
- Conclusion:
- The tutorial concludes by highlighting the significance of spectra in understanding the behavior of electromagnetic radiation and the unique features displayed by different elements.
This comprehensive tutorial provides a thorough exploration of spectra, from their fundamental definitions to real-world examples, offering a valuable resource for those delving into the intricate world of electromagnetic radiation.
