Voltaic or Galvanic Cell
An electrochemical cell that converts the chemical energy of redox reactions into electrical energy is called a galvanic cell or a voltaic cell. The cell uses spontaneous redox reactions to create an electrical current. It consists of two separate half-cells. A half-cell is made up of an electrode (a strip of metal, M) within an option including Mn+ ions in which M is any approximate metal.
In oxidation-reduction reactions, electrons are moved from one species to another species. Energy is released if the reaction happens spontaneously. For that reason, the released energy is used to do helpful work. To tackle this energy, it is required to split the reaction into two different half-reactions viz. oxidation and reduction.
With the help of two various containers and wire, the reactions are taken into them to drive the electrons from one end to the other end. This produces a voltaic cell.
Working of Voltaic or Galvanic Cell
A voltaic or a galvanic cell consists of two half-cells that are electrically connected. Each half cell is a portion of the total cell in which a half-reaction occurs. The left half-cell consists of a strip of zinc metal dipped in 1.0 M solution of zinc sulfate providing the following balance:
Zn(s) → Zn (aq)+2e–
The right half-cell is a copper metal strip that dips into 1.0 M copper sulfate solution and the equilibrium here is represented as follows:
Cu(s) → Cu2++2e–
These half-cells are connected electrically by a salt bridge. If the solutions were to blend, direct chemical reactions would occur, destroying the half-cells. The salt bridge contains a liquid solution of potassium chloride in a gel. Zinc tends to lose electrons quicker than copper.
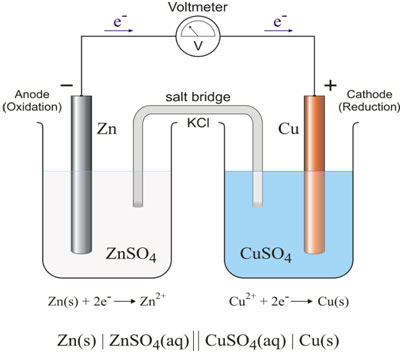
Zn electrode takes on a negative charge relative to the copper electrode. If the external circuit is nearby connecting the two electrodes, electrons flow from the zinc through the external circuit to the copper electrode. The following half-cell reactions occur at two electrodes and cell potential at standard conditions is 1.1 volts. It is denoted by E °.
At anode Zn (s)→ Zn2+ (aq) +2e–(Oxidation)
At cathode Cu2+(aq) +2e–→ Cu(s)(Reduction)
The overall voltaic cell reaction is the amount of these two half-cell reactions.
Zn (s) + Cu 2+ (aq) → Zn2+ (aq) + Cu (s)E =1.1V
This voltaic cell can be represented as follows;
Zn(s) /Zn 2+(aq) 1M ǁ Cu 2+ +1M(aq)/Cu(s)E =1.1V
The signǁrepresents the salt bridge.
The function of Salt Bridge
Considering that, zinc ions are produced as electrons leave the anode, we have a process that tends to produce a net positive charge in the left beaker. Actually, the concentration of Zn2+ ions increases in the left beaker. Likewise, the arrival of the electrons at the copper cathode and their reaction with copper ions tend to produce a net negative charge in the right beaker.
The purpose of the salt bridge is to prevent any net charge accumulation in either beaker by allowing negative ions to leave the right beaker, diffuse through the bridge and get in the left beaker. If this diffusional exchange of ions does not happen, the net charge building up in the beakers would instantly stop the flow of electrons through the external circuit and the oxidation-reduction reaction would stop.
Lots of other oxidation-reduction reactions can be carried out effectively in galvanic cells using different electrodes. It is natural to consider these cell procedures as separated into 2 half-reactions which occur at the two electrodes. In a voltaic cell, the electric current in the external circuit can be utilized to light a bulb, drive a motor, and so on.
Voltaic Cell is Reversible Cell
On the other hand, if the external circuit is replaced by a source of electricity that opposes the voltaic cell, the electrode reactions can be reversed. Now, the external source presses the electrons in the opposite direction and provides energy or work to the cell so that the reverse nonspontaneous reaction occurs. Such, a cell is called a reversible cell. For the zinc-copper cell, the half-cell reactions are reversed to give.
Zn2+ (aq)+2e–→ Zn(s)(reduction)
Cu(s)→ Cu2+ +2e–(oxidation)
and the overall reaction is reversed, ends up being
Zn2+ (aq) + Cu(s)→ Zn(s) + Cu2+ (aq)
Oxidation takes place at the copper electrode and reduction takes place at the zinc electrode and the cell operates as an electrolytic cell in which energy from an external source drives a nonspontaneous reaction.
When a cell operates as a voltaic the electrode at which reduction happens is called the cathode while the electrode at which oxidation takes place is called the anode. Hence in a voltaic cell, Zn serves as an anode and Cu serves as a cathode.
MCQs
- Question 1: What is a voltaic cell also known as?
- A) Galvanic cell
- B) Electrolytic cell
- C) Thermoelectric cell
- D) Photoelectric cell
- Answer: A) Galvanic cell
- Question 2: What type of energy does a voltaic cell convert?
- A) Mechanical energy
- B) Chemical energy
- C) Thermal energy
- D) Nuclear energy
- Answer: B) Chemical energy
- Question 3: In a voltaic cell, what is the left half-cell typically made of?
- A) Platinum electrode
- B) Zinc metal electrode
- C) Copper metal electrode
- D) Graphite electrode
- Answer: B) Zinc metal electrode
- Question 4: What happens in the salt bridge of a voltaic cell?
- A) It facilitates the flow of electrons.
- B) It prevents any net charge accumulation.
- C) It generates electrical energy.
- D) It initiates redox reactions.
- Answer: B) It prevents any net charge accumulation.
- Question 5: What is the purpose of splitting a redox reaction into two half-reactions in a voltaic cell?
- A) To complicate the process
- B) To generate heat
- C) To release energy
- D) To do useful work
- Answer: D) To do useful work
- Question 6: What is the overall cell potential of a voltaic cell at standard conditions denoted by?
- A) E+
- B) E-
- C) E*
- D) E°
- Answer: D) E°
- Question 7: Which electrode is considered the cathode in a voltaic cell?
- A) Zinc electrode
- B) Copper electrode
- C) Platinum electrode
- D) Graphite electrode
- Answer: B) Copper electrode
- Question 8: What is the purpose of the salt bridge in a voltaic cell?
- A) To generate electrical energy
- B) To store charge
- C) To prevent charge accumulation
- D) To increase cell potential
- Answer: C) To prevent charge accumulation
- Question 9: In the voltaic cell reaction Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s), what is oxidized?
- A) Zn
- B) Cu
- C) Zn²⁺
- D) Cu²⁺
- Answer: A) Zn
- Question 10: What drives the flow of electrons in a voltaic cell?
- A) Chemical reactions
- B) Electric field
- C) Salt bridge
- D) External circuit
- Answer: D) External circuit
- Question 11: What is the function of the salt bridge in preventing charge accumulation?
- A) Facilitates electron flow
- B) Releases ions into the solution
- C) Diffuses negative ions
- D) Initiates redox reactions
- Answer: C) Diffuses negative ions
- Question 12: When a voltaic cell operates as an electrolytic cell, what drives the nonspontaneous reaction?
- A) Chemical reactions
- B) Salt bridge
- C) External source of electricity
- D) Redox reactions
- Answer: C) External source of electricity
- Question 13: Which type of cell involves a spontaneous redox reaction?
- A) Electrolytic cell
- B) Thermoelectric cell
- C) Voltaic cell
- D) Photoelectric cell
- Answer: C) Voltaic cell
- Question 14: What is the overall reaction when a voltaic cell operates at standard conditions?
- A) Zn(s) → Zn²⁺(aq) + 2e⁻
- B) Cu²⁺(aq) + 2e⁻ → Cu(s)
- C) Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
- D) Zn²⁺(aq) + Cu(s) → Zn(s) + Cu²⁺(aq)
- Answer: C) Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
- Question 15: What is the purpose of an external source in a reversible cell?
- A) To store energy
- B) To drive spontaneous reactions
- C) To oppose voltaic cell reactions
- D) To facilitate charge accumulation
- Answer: C) To oppose voltaic cell reactions
Summary
In conclusion, a Voltaic or Galvanic Cell is an electrochemical cell that transforms the chemical energy of redox reactions into electrical energy. This process involves spontaneous redox reactions occurring in two separate half-cells, each containing an electrode and an electrolyte solution. The oxidation-reduction reactions in these half-cells release energy, which is harnessed to perform useful work.
The working of a Voltaic Cell involves the connection of two half-cells through an external circuit, allowing electrons to flow from one electrode to another. This creates an electrical current, and the overall cell potential at standard conditions is denoted by E°.
The salt bridge plays a crucial role in preventing net charge accumulation in the half-cells. It allows negative ions to diffuse between the half-cells, maintaining charge balance and ensuring the continuous flow of electrons through the external circuit.
Additionally, a Voltaic Cell can operate as a reversible cell if the external circuit is replaced by a source of electricity opposing the voltaic cell. In this case, the electrode reactions are reversed, and the cell functions as an electrolytic cell, where an external source drives a nonspontaneous reaction.
Overall, the voltaic cell offers a practical way to convert chemical energy into electrical energy, with applications ranging from lighting bulbs to driving motors. Understanding the principles of voltaic cells is fundamental to comprehending electrochemical processes and their diverse applications.
