Enzymes – The Biocatalyst
Enzymes are the most important group of proteins that are biologically active.
They greatly increase the efficiency of biochemical response and are specific for each type of reaction.
Without these enzymes, the reaction would continue at a very sluggish speed making life difficult.
- 1) Structure of Enzymes
- 2) Co-Factor
- 3) Activator
- 4) Prosthetic group
- 5) Coenzyme
- 6) Apoenzyme and Holoenzyme
- 7) Presence of Enzymes
- 8) Characteristics of Enzymes
- 9) Mechanism of Enzyme Action
- 10) Summary
- 11) Enzymes – The Biocatalyst: Multiple-Choice Questions (MCQs) with Answers
- 12) Frequently Asked Questions (FAQs) – Enzymes – The Biocatalyst Tutorial
- 13) Wrapping up Enzymes tutorial
- 14) You may also like to learn:
Structure of Enzymes
Enzymes are made up of hundreds of amino acids collaborated and coiled upon themselves to form a globular structure. The catalytic activity is limited to a small portion of the structure called the active site. The reactant called substrate is connected to the active site including only a few amino acids, while the rest of the bulk of the amino acids maintains the globular structure of the enzyme.

Co-Factor
Some enzymes consist exclusively of proteins. Others also have a non-protein part, which is essential for the proper functioning of the enzymes. This non-protein part is called the co-factor.
Function of co-factor
The cofactor usually serves as a “bridge” in between the enzyme and its substrate, often it contributes straight to the chemical reactions which bring about catalysis. Sometimes the co-factor offers a source of chemical energy, assisting to drive reactions that would otherwise be difficult or impossible.
Activator
Some enzymes utilize metal ions as co-factors like Mg2+, Fe2+, Cu2+, Zn2+, etc. The separable co-factor is known as an activator if it is an inorganic ion.

Prosthetic group
f the non-protein part is covalently bonded, it is known as a prosthetic group. Examples: thiamine pyrophosphate, pyridoxal-phosphate, and biotin.
Coenzyme
If it is loosely attached to the protein part, it is called a coenzyme. Examples: nicotinamide adenine dinucleotide (NAD), nicotine amide adenine dinucleotide phosphate (NADP), and flavin adenine dinucleotide (FAD).
Apoenzyme and Holoenzyme
An enzyme with its coenzyme, or prosthetic group, eliminated is designated as apoenzyme. Adding the properly concentrated coenzyme to the apoenzyme will bring back enzyme activity. A triggered enzyme including a polypeptide chain and a cofactor is called a holoenzyme.
Presence of Enzymes
Numerous enzymes are just dissolved in the cytoplasm. Other enzymes are firmly bound to certain subcellular organelles. They are produced by living cells for use in or near the site of their production. The enzymes essential in photosynthesis are discovered in the chloroplasts and enzymes involved in cellular respiration are found in the mitochondria. Some of the enzymes which are associated with the synthesis of proteins are an essential part of ribosomes.
A lot of enzymes do not float about in a sort of cytoplasmic soup’ but are connected to membrane systems inside the cell in specific and organized arrangements.
Mitochondria and chloroplasts are good examples of this.
Characteristics of Enzymes
Enzymes, the biochemical catalysts possess the following important attributes.
- All enzymes are globular proteins.
- They increase the rate of a reaction without themselves being consumed.
- Their existence does not affect the nature or properties of the final product.
- Percentages of an enzyme can speed up chain reactions.
- They are extremely specific in their action; a single enzyme catalyzes just a single chemical reaction or a group of associated reactions.
- They are sensitive to even a minor change in pH, temperature level, and substrate concentration.
- Some enzymes require a co-factor for their proper performance.
- They lower the activation energy of the reactions.
Mechanism of Enzyme Action
An enzyme is a 3-dimensional globular protein that has specific chemical composition due to its element amino acids and a specific shape. Every enzyme by virtue of its specificity acknowledges and reacts with a unique chemical compound called the substrate. Any enzyme, therefore, responds only with its specific substrate and transforms it into product(s). It is then released unaltered and therefore can be used again and again.
E + S ⇌ ES ⇌ E + P
Enzyme Substrate Enzyme Substrate Complex Enzyme Product
In specific cases, enzymes act in a series of chain reactions in a specific order to complete a metabolic path such as respiration or photosynthesis. The succeeding enzymes including these reactions are usually present together in a precise order of reaction such that substrate molecules can be literally handed on from one enzyme to another forming an enzyme to enzyme chain. In this way, the products from one step in the pathway are moved to the enzyme catalysing the next action.
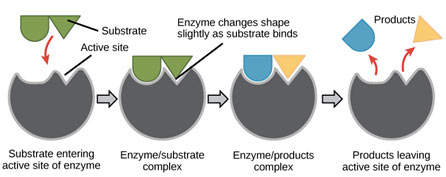
An enzyme and its substrate react with each other through a definite charge-bearing site of an enzyme called the active site. The charge and shape of the active site are formed by some amino acids present in the polypeptide chain of the active site of the enzyme. These amino acids are brought more detailed and are set up in a particular way by coiling and folding of the polypeptide chain within the globular symmetry of the enzyme.
The active website of the enzyme is comprised of 2 definite regions i.e. the binding site and the catalytic site. The binding site helps the enzyme in the recognition and binding of a proper substrate to produce an ES complex. This reaction triggers the catalytic site. Catalytic site catalyzes the change of the substrate into product(s). Hence the enzyme after catalysis removes itself from the products unchanged. The enzyme requires liquid medium for its activity.
Summary
An enzyme is a biological catalyst that can accelerate a specific chemical reaction by lowering the activation energy but remain unaltered in the reaction. Most enzymes are protein but some are nucleic acids like ribozymes.
Enzymes have enormous catalytic power. They greatly increase the rate at which chemical reactions take place. These are three-dimensional globular molecules. It has at least one surface region. This region has crevice. This crevice is known as the enzyme’s active site.
The substrate molecule fits into it in a very specific way. The metal ions loosely attached to enzymes are cofactors. These are required for the proper functioning of enzymes.
Coenzymes are non-protein, organic molecules that participate in enzyme-catalyzed reaction. Many vitamins like niacin and riboflavin function as coenzymes. Coenzymes transport energy in the form of hydrogen atoms from one enzyme to another.
Enzymes – The Biocatalyst: Multiple-Choice Questions (MCQs) with Answers
- What is the primary function of enzymes in biological systems?
- A. Structural support
- B. Energy storage
- C. Biocatalysis
- D. Oxygen transport
- Answer: C. Biocatalysis
- How are enzymes structured?
- A. Linear chains
- B. Coiled polymers
- C. Planar sheets
- D. Branched networks
- Answer: B. Coiled polymers
- Which part of the enzyme is responsible for catalytic activity?
- A. Globular structure
- B. Active site
- C. Amino acid chain
- D. Substrate-binding region
- Answer: B. Active site
- What is the function of a cofactor in enzyme activity?
- A. Catalytic site recognition
- B. Substrate binding
- C. Structural support
- D. Bridge between enzyme and substrate
- Answer: D. Bridge between enzyme and substrate
- Which term is used for an inorganic ion that serves as an activator in enzymatic reactions?
- A. Substrate
- B. Cofactor
- C. Prosthetic group
- D. Activator
- Answer: D. Activator
- When a non-protein part is covalently bonded to an enzyme, what is it called?
- A. Cofactor
- B. Prosthetic group
- C. Activator
- D. Coenzyme
- Answer: B. Prosthetic group
- What distinguishes a coenzyme from a prosthetic group?
- A. Attachment strength
- B. Chemical composition
- C. Size
- D. Location in the enzyme
- Answer: A. Attachment strength
- What is an enzyme without its coenzyme or prosthetic group called?
- A. Substrate
- B. Apoenzyme
- C. Holoenzyme
- D. Cofactor
- Answer: B. Apoenzyme
- Which organelle is a common site for the presence of enzymes involved in photosynthesis?
- A. Ribosomes
- B. Mitochondria
- C. Nucleus
- D. Chloroplasts
- Answer: D. Chloroplasts
- What is a characteristic feature of enzymes in terms of their action on reactions?
- A. High specificity
- B. Generalized function
- C. Low sensitivity to pH changes
- D. Limited substrate compatibility
- Answer: A. High specificity
- How do enzymes affect the activation energy of reactions?
- A. Increase it
- B. Decrease it
- C. Remain unchanged
- D. Neutralize it
- Answer: B. Decrease it
- Which factor does NOT influence enzyme activity?
- A. pH
- B. Temperature
- C. Substrate concentration
- D. Product concentration
- Answer: D. Product concentration
- In enzyme action, what is the term for the reactant that interacts with the enzyme?
- A. Product
- B. Substrate
- C. Coenzyme
- D. Activator
- Answer: B. Substrate
- What type of reaction is catalyzed by enzymes in a metabolic pathway?
- A. Independent reactions
- B. Parallel reactions
- C. Random reactions
- D. Sequential reactions
- Answer: D. Sequential reactions
- What is the product of an enzyme-substrate reaction?
- A. Enzyme
- B. Substrate
- C. Complex
- D. Product
- Answer: D. Product
- What is the role of the active site’s binding region in enzyme action?
- A. Catalysis
- B. Substrate recognition
- C. Product formation
- D. Cofactor binding
- Answer: B. Substrate recognition
- What is the overall result of an enzyme catalyzing a reaction?
- A. Enzyme is consumed
- B. Substrate is consumed
- C. Enzyme is unchanged
- D. Substrate is unchanged
- Answer: C. Enzyme is unchanged
- What type of medium does an enzyme require for its activity?
- A. Solid
- B. Gas
- C. Liquid
- D. Plasma
- Answer: C. Liquid
- What is the term for an enzyme without its coenzyme or prosthetic group?
- A. Apoenzyme
- B. Holoenzyme
- C. Proenzyme
- D. Catalytic enzyme
- Answer: A. Apoenzyme
- In the mechanism of enzyme action, what happens during the formation of an ES complex?
- A. Catalysis
- B. Substrate binding
- C. Product release
- D. Enzyme denaturation
- Answer: B. Substrate binding
- What role does the catalytic site play in enzyme action?
- A. Substrate recognition
- B. Product formation
- C. Cofactor binding
- D. Enzyme stabilization
- Answer: B. Product formation
Frequently Asked Questions (FAQs) – Enzymes – The Biocatalyst Tutorial
1. What is the significance of enzymes in biological systems?
- Enzymes play a crucial role as biocatalysts, enhancing the efficiency of biochemical reactions and exhibiting specificity for each type of reaction.
2. How are enzymes structured, and what is the active site?
- Enzymes are composed of coiled amino acids, forming a globular structure. The active site, a small portion of the structure, is where catalytic activity occurs.
3. What is a co-factor in the context of enzymes?
- Some enzymes have a non-protein part called a co-factor, which is essential for proper enzyme functioning.
4. How does a co-factor contribute to enzyme activity?
- The co-factor often acts as a “bridge” between the enzyme and its substrate, contributing directly to the chemical reactions leading to catalysis.
5. What is the role of an activator in enzymatic reactions?
- Activators, often inorganic ions like Mg2+, Fe2+, etc., serve as separable co-factors that enhance enzyme activity.
6. What is a prosthetic group, and how is it different from a coenzyme?
- If the non-protein part is covalently bonded, it is a prosthetic group. Coenzymes are loosely attached to the protein part of enzymes.
7. Explain the terms Apoenzyme and Holoenzyme.
- An enzyme without its coenzyme or prosthetic group is an apoenzyme. Adding the proper coenzyme restores enzyme activity. A holoenzyme is an activated enzyme with its cofactor.
8. Where are enzymes located within cells?
- Enzymes can be dissolved in the cytoplasm or tightly bound to subcellular organelles. Their location depends on their function.
9. What are the key characteristics of enzymes?
- Enzymes are globular proteins, accelerate reactions without being consumed, exhibit high specificity, and are sensitive to changes in pH, temperature, and substrate concentration.
10. How do enzymes influence the activation energy of reactions?
- Enzymes lower the activation energy of reactions, making them more favorable and efficient.
11. Can enzymes speed up chain reactions? – Yes, enzymes can accelerate chain reactions, contributing to the efficiency of biochemical pathways.
12. Do enzymes affect the nature or properties of the final product? – No, the presence of enzymes does not alter the nature or properties of the final product.
13. What is the mechanism of enzyme action? – Enzymes interact with specific substrates through an active site, forming an enzyme-substrate complex (ES complex) that undergoes catalysis to produce products.
14. How do enzymes act in metabolic pathways? – Enzymes in metabolic pathways act sequentially, with substrates handed from one enzyme to another in an orderly chain of reactions.
15. What is the role of the active site in enzyme-substrate interactions? – The active site consists of binding and catalytic regions. It facilitates substrate recognition, forming the ES complex that undergoes catalysis.
16. Why do enzymes require a liquid medium for activity? – Enzymes need a liquid medium for proper conformation, substrate recognition, and catalytic action within their active sites.
Wrapping up Enzymes tutorial
Enzymes, vital proteins in biological systems, play a pivotal role in enhancing biochemical reactions’ efficiency, ensuring specificity for each reaction type. This tutorial delves into the structure, co-factors, activation, and characteristics of enzymes, shedding light on their indispensable functions.
- Structure of Enzymes: Enzymes consist of coiled amino acids, forming a globular structure. The catalytic activity resides in the active site, a small portion of the structure, where substrates bind for reactions.
- Co-Factor and Activator: Some enzymes exclusively comprise proteins, while others include a non-protein component called a co-factor. Co-factors act as bridges, contributing to catalysis. Metal ions can serve as activators if they are inorganic ions.
- Prosthetic Group and Coenzyme: If the non-protein part is covalently bonded, it is a prosthetic group. Coenzymes, loosely attached to the protein, play essential roles. Examples include thiamine pyrophosphate and nicotinamide adenine dinucleotide (NAD).
- Apoenzyme and Holoenzyme: Apoenzyme refers to an enzyme without its coenzyme or prosthetic group. Adding the proper coenzyme restores enzyme activity. A holoenzyme is the activated form, including a polypeptide chain and a cofactor.
- Presence of Enzymes: Enzymes may be dissolved in the cytoplasm or bound to subcellular organelles, with locations based on their functions. Examples include enzymes in chloroplasts for photosynthesis and mitochondria for cellular respiration.
- Characteristics of Enzymes: Enzymes, being globular proteins, accelerate reactions without being consumed, exhibit specificity, and are sensitive to changes in pH, temperature, and substrate concentration. They often require co-factors and lower activation energy.
- Mechanism of Enzyme Action: Enzymes are 3D globular proteins with specific active sites. They react with substrates, forming an enzyme-substrate complex (ES complex) that undergoes catalysis, leading to the production of products. Enzymes act sequentially in metabolic pathways.
This comprehensive tutorial provides insights into the intricate world of enzymes, highlighting their structural intricacies, functional aspects, and vital roles in biochemical processes. Understanding these aspects is crucial for appreciating the complexity of enzymatic reactions in living organisms.
