Reactions of Lipids
As we have discussed lipids in the previous article. In this article, we will discuss only the reactions of lipids and tests for the purity of fats in lipids.
- 1) Saponification
- 2) Hydrogenation
- 3) Peroxidation
- 4) Rancidity
- 5) Saponification Number
- 6) Iodine Number
- 7) Acid Number
- 8) Reichert Meissl Number
- 9) MCQs
- 10) Frequently Asked Questions (FAQs) – Reactions of Lipids
- 11) Summary: Reactions of Lipids – Saponification, Hydrogenation, Peroxidation, Rancidity
- 12) You may also like to learn:
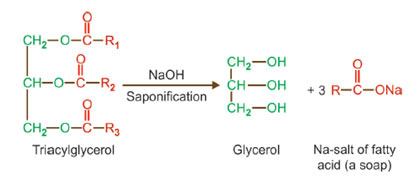
Saponification
Hydrolysis of fat by alkali is called saponification. The products are glycerol and the alkali salts of the fatty acids, which are called soaps. Acid hydrolysis of fat yields free fats and glycerol.
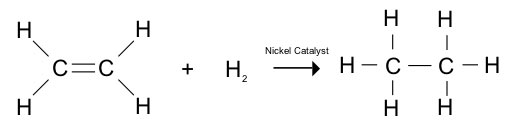
Hydrogenation
Hydrogenation of unsaturated fats in the presence of a catalyst (nickel) is referred to as “hardening”. It is commercially important as a method of converting these liquid fats, usually of plant origin into solid fats as margarine, vegetable ghee, and so on.
Peroxidation
Peroxidation (auto-oxidation) of lipids exposed to oxygen is accountable not just for deterioration of foods (rancidity) however also for damage to tissues in vivo, where it might be a cause of cancer. Lipid peroxidation is a domino effect creating complimentary radicals that initiate further peroxidation.
To manage and lower peroxidation, people utilize anti-oxidants. Naturally occurring anti-oxidants consist of vitamin E (tocopherol) and β-carotene (provitamin A), which are lipid-soluble, and vitamin C, which is water-soluble.
Rancidity
The undesirable smell and taste, developed by natural fats upon aging, is described as “rancidity”. Rancidity might be because of hydrolysis or oxidation of fat.
Rancidity due to hydrolysis
Naturally occurring fats, particularly those from animal sources, are polluted with enzyme lipase. The action of lipase brings about partial hydrolysis of glycerides of fat.
– Rancidity may likewise be brought on by different oxidative procedures. For example, oxidation at the double bonds of unsaturated fats of glycerides may form peroxides, which then disintegrate to form aldehydes of unpleasant odor and taste, this process is increased by exposure to light or heat.
Lots of natural vegetable fats and oils may include antioxidants like vitamin E which avoid the onset of rancidity. Therefore, vegetable fats can be preserved for a longer time than animal fats.
Tests for the purity of Fats
Fats are identified and their purity assayed by the following tests:
Saponification Number
It is specified as, number of mgs of KOH required to saponify one gm of fat. It is inversely proportional to the molecular weight of fat. This value is high in fats including a short-chain fatty acid. For example, the saponification number of:
– Butter = 220
– Coconut oil = 260
Iodine Number
The number of grams of iodine required to saturate 100 grams of a provided fat is called iodine number. Considering that iodine is taken up by the double bonds, a high iodine number shows a high degree of unsaturation of the fatty acids in fat, e.g.
– Butter fat = 27
– Coconut oil = 8
– Linseed oil = 200
Iodine number is important in the identification of the fat or oil in addition to is utilized for recognition of adulteration of oils. This unsaturation remains in the form of double bonds which react with iodine substances. The greater the iodine value, the more unsaturated fatty acid bonds are present in fat.
Acid Number
The number of mg of KOH required to reduce the effects of the free fatty acids present in one gm of fat is called acid number. The acid number indicates the degree of rancidity of the given fat. The acid number is directly proportional to the rancidity. The edibility of fat is inversely proportional to the acid number. Refined oil should not consist of totally free fatty acids. The existence of free fatty acids in any oil suggests that it is not pure.
Reichert Meissl Number
The number of ml of 0.1 N alkali, needed to reduce the effects of the unstable fatty acids distilled from 5 gm of fat. For example, the Reichert Meissl value for:
– Butter = 26
– Coconut oil = 7
It is less than one for other edible oils. The admixture of certain fatty acids may be utilized to prepare synthetic butter which might imitate butter in the majority of the constants except for RM value and for this reason, can be detected.
MCQs
- Question 1: What is saponification?
- A) Hydrolysis of fat by acid
- B) Hydrolysis of fat by alkali
- C) Hydrogenation of fat
- D) Peroxidation of lipids
- Answer: B
- Question 2: What are the products of saponification?
- A) Glycerol and fatty acids
- B) Alkali salts of fatty acids and glycerol
- C) Glycerol and alkali
- D) Alkali salts and water
- Answer: B
- Question 3: What is the commercial importance of hydrogenation?
- A) Formation of soap
- B) Conversion of liquid fats into solid fats
- C) Peroxidation prevention
- D) Rancidity reduction
- Answer: B
- Question 4: What causes rancidity in lipids?
- A) Hydrolysis
- B) Peroxidation
- C) Both A and B
- D) None of the above
- Answer: C
- Question 5: Which catalyst is used in hydrogenation?
- Question 6: What is peroxidation also known as?
- A) Oxidation
- B) Auto-oxidation
- C) Hydrolysis
- D) Hydrogenation
- Answer: B
- Question 7: What initiates further peroxidation in lipids?
- A) Antioxidants
- B) Free radicals
- C) Aldehydes
- D) Glycerol
- Answer: B
- Question 8: What is the undesirable smell and taste in aged fats referred to as?
- A) Oxidation
- B) Saponification
- C) Rancidity
- D) Hydrogenation
- Answer: C
- Question 9: What prevents the onset of rancidity in vegetable fats?
- A) Lipase
- B) Peroxides
- C) Antioxidants like vitamin E
- D) Aldehydes
- Answer: C
- Question 10: How is the saponification number defined?
- A) Number of mgs of KOH required to neutralize free fatty acids
- B) Number of mgs of KOH required to saponify one gm of fat
- C) Number of grams of iodine required to saturate 100 grams of fat
- D) Number of ml of 0.1 N alkali needed to neutralize volatile fatty acids
- Answer: B
- Question 11: What does the iodine number indicate?
- A) Degree of rancidity
- B) Degree of unsaturation of fatty acids
- C) Degree of saturation of fatty acids
- D) Degree of peroxidation
- Answer: B
- Question 12: What does the acid number indicate?
- A) Degree of rancidity
- B) Degree of unsaturation
- C) Degree of peroxidation
- D) Degree of hydrogenation
- Answer: A
- Question 13: Which test helps identify the fat or oil and detect adulteration?
- A) Saponification Number
- B) Iodine Number
- C) Acid Number
- D) Reichert Meissl Number
- Answer: B
- Question 14: What does the Reichert Meissl Number measure?
- A) Degree of hydrogenation
- B) Degree of unsaturation
- C) Degree of acidity
- D) Degree of purity
- Answer: B
- Question 15: What is the Reichert Meissl value for coconut oil?
- A) 7
- B) 26
- C) 8
- D) 220
- Answer: A
- Question 16: Which test is inversely proportional to the molecular weight of fat?
- A) Saponification Number
- B) Iodine Number
- C) Acid Number
- D) Reichert Meissl Number
- Answer: A
- Question 17: What is the primary cause of lipid peroxidation?
- A) Antioxidants
- B) Hydrogenation
- C) Peroxidation
- D) Saponification
- Answer: C
- Question 18: What initiates peroxidation in lipids?
- A) Antioxidants
- B) Free radicals
- C) Aldehydes
- D) Glycerol
- Answer: B
- Question 19: What is the commercial significance of saponification?
- A) Formation of solid fats
- B) Formation of soaps
- C) Prevention of rancidity
- D) Peroxidation reduction
- Answer: B
- Question 20: Which element is taken up by the double bonds in iodine number determination?
- Question 21: What does a high iodine number indicate?
- A) High rancidity
- B) High unsaturation of fatty acids
- C) High saturation of fatty acids
- D) High hydrogenation
- Answer: B
Frequently Asked Questions (FAQs) – Reactions of Lipids
- What are the main reactions of lipids discussed in this tutorial?
- The main reactions discussed are Saponification, Hydrogenation, Peroxidation, and Rancidity.
- Define Saponification and its products.
- Saponification is the hydrolysis of fat by alkali, resulting in glycerol and alkali salts of fatty acids, commonly known as soaps.
- Why is hydrogenation commercially important, and what catalyst is used?
- Hydrogenation converts unsaturated fats into solid fats (hardening), and nickel is used as a catalyst.
- Explain Peroxidation and its significance.
- Peroxidation (auto-oxidation) of lipids, exposed to oxygen, causes rancidity in foods and may contribute to tissue damage, potentially leading to cancer.
- What are some natural antioxidants to manage peroxidation?
- Natural antioxidants include vitamin E (tocopherol), β-carotene (provitamin A), and vitamin C.
- Define Rancidity and explain its causes.
- Rancidity is the undesirable smell and taste in aged fats, caused by hydrolysis or oxidation of fat. Lipase and oxidative processes contribute to rancidity.
- How can natural vegetable fats be preserved longer than animal fats against rancidity?
- Vegetable fats may contain antioxidants like vitamin E, delaying the onset of rancidity.
- What tests are used to determine the purity of fats?
- Tests for purity include Saponification Number, Iodine Number, Acid Number, and Reichert Meissl Number.
- What is the Saponification Number, and what does it indicate?
- The Saponification Number is the mgs of KOH required to saponify one gm of fat, inversely proportional to the molecular weight of fat.
- Explain the Iodine Number and its importance.
- Iodine Number is the grams of iodine required to saturate 100 grams of fat, indicating the degree of unsaturation. It aids in identifying fats and detecting adulteration.
- Define Acid Number and its relationship with the degree of rancidity.
- Acid Number is the mg of KOH required to neutralize free fatty acids in one gm of fat. It is directly proportional to the degree of rancidity.
- What does Reichert Meissl Number measure, and how is it used for detection?
- Reichert Meissl Number measures the ml of 0.1 N alkali needed to neutralize volatile fatty acids. It helps in detecting synthetic butter through constants like RM value.
Summary: Reactions of Lipids – Saponification, Hydrogenation, Peroxidation, Rancidity
This tutorial delves into crucial reactions of lipids and methods for assessing the purity of fats. The main reactions covered are:
- Saponification:
- Defined as the hydrolysis of fat by alkali, yielding glycerol and alkali salts of fatty acids (soaps).
- Acid hydrolysis produces free fats and glycerol.
- Hydrogenation:
- Involves the hardening of unsaturated fats using a nickel catalyst.
- Commercially important for converting liquid plant fats into solid fats like margarine.
- Peroxidation:
- Auto-oxidation of lipids exposed to oxygen, causing food deterioration (rancidity) and potential tissue damage.
- Managed by antioxidants such as vitamin E, β-carotene, and vitamin C.
- Rancidity:
- Describes the undesirable smell and taste in aged natural fats.
- Can result from hydrolysis or oxidation of fats.
- Enzyme lipase in animal fats and oxidative processes contribute to rancidity.
- Tests for Purity:
- Saponification Number:
- Inversely proportional to the molecular weight of fat.
- Higher values for fats with short-chain fatty acids.
- Iodine Number:
- Indicates the degree of unsaturation in fatty acids.
- Higher values suggest more unsaturated bonds.
- Acid Number:
- Directly proportional to the degree of rancidity.
- Indicates edibility; refined oil should have low acid numbers.
- Reichert Meissl Number:
- Measures ml of 0.1 N alkali to neutralize volatile fatty acids.
- Differentiates butter from other edible oils based on constants like RM value.
- Saponification Number:
Understanding these reactions and tests is essential for comprehending the behavior and quality of lipids and their derivatives.