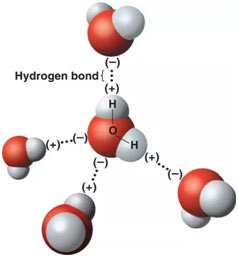
A Hydrogen Bond
A hydrogen bond is an electromagnetic attraction produced between a partially positively charged hydrogen atom attached to a highly electronegative atom and another nearby electronegative atom.
A hydrogen bond is a kind of dipole-dipole interaction; it is not a true chemical bond.
These attractions can occur between molecules (intermolecularly) or within various parts of a single molecule (intramolecularly).
- 1) Formation of Hydrogen Bond
- 2) Hydrogen Bond Donor
- 3) Hydrogen Bond Acceptor
- 4) Strength of the Hydrogen bond
- 5) Examples of Hydrogen Bonding
- 6) Applications for Hydrogen Bonds
- 7) Multiple-Choice Questions (MCQs) with Answers:
- 8) Frequently Asked Questions (FAQs):
- 9) Summary:
- 10) You may also like to learn:
Formation of Hydrogen Bond
In molecules consisting of N-H, O-H, or F-H bonds, the big difference in electronegativity between the H atom and the N, O or F atom leads to a highly polar covalent bond (i.e., a bond dipole).
Because of the distinction in electronegativity, the H atom bears a large partial positive charge and the N, O, or F atom bears a high partial negative charge. Thus, partially different charges bring in each other, and a hydrogen bond is formed.

Hydrogen Bond Donor
A hydrogen atom attached to a fairly electronegative atom is a hydrogen bond donor. This electronegative atom is typically fluorine, oxygen, or nitrogen. The electronegative atom attracts the electron cloud from the hydrogen nucleus and, by decentralizing the cloud, leaves the hydrogen atom with a positive partial charge.
Because of the small size of hydrogen relative to other atoms and molecules, the resulting charge, though only partial, is stronger. In the molecule of ethanol, there is one hydrogen atom bonded to an oxygen atom, which is very electronegative. This hydrogen atom is a hydrogen bond donor.
Hydrogen Bond Acceptor
A hydrogen bond results when this strong partial positive charge draws in a lone pair of electrons from another atom, which ends up being the hydrogen bond acceptor. An electronegative atom such as fluorine, oxygen, or nitrogen is a hydrogen bond acceptor, no matter whether it is bonded to a hydrogen atom or not.
Greater electronegativity of the hydrogen bond acceptor will develop a more powerful hydrogen bond. The diethyl ether molecule includes an oxygen atom that is not bonded to a hydrogen atom, making it a hydrogen bond acceptor.
Strength of the Hydrogen bond
The hydrogen bond is a weak bond. The strength of the hydrogen bond is in-between the weak van der Waals forces and the strong covalent bonds.
The dissociation energy of the hydrogen bond depends upon the attraction of the shared pair of electrons and for this reason on the electronegativity of the atom.
Examples of Hydrogen Bonding
Hydrogen Bonding in Hydrogen fluoride
Fluorine having the greatest value of electronegativity forms the strongest hydrogen bond.

Hydrogen Bonding in Water
A water molecule contains a highly electronegative oxygen atom linked to the hydrogen atom. Oxygen atom draws in the shared pair of electrons more and this end of the molecule becomes negative whereas the hydrogen atoms end up being positive.

Hydrogen Bonding in Ammonia
It consists of highly electronegative atom nitrogen linked to hydrogen atoms.

Hydrogen Bond in Alcohols and Carboxylic acid
Alcohol is a kind of organic molecule which includes an -OH group. Usually, if any molecule which includes the hydrogen atom is connected to either oxygen or nitrogen directly, then hydrogen bonding is quickly formed.

Properties of Hydrogen Bonding
- Solubility: Lower alcohols are soluble in water because of the hydrogen bonding which can take place between water and alcohol molecule.
- Volatility: As the compounds including hydrogen bonding in between various particles have a greater boiling point, so they are less unstable.
- Viscosity and surface area tension: The substances which consist of hydrogen bonding exists as an associated molecule. So, their circulation ends up being comparatively difficult. They have higher viscosity and high surface tension.
Applications for Hydrogen Bonds
- Hydrogen bonds take place in inorganic molecules, such as water, and organic molecules, such as DNA and proteins. The two complementary strands of DNA are held together by hydrogen bonds in between complementary nucleotides (A&T, C&G). Hydrogen bonding in the water adds to its unique properties, including its high boiling point (100 ° C) and surface area tension.
- The hydrogen bonds formed in between water molecules in water drops are more powerful than the other intermolecular forces between the water molecules and the leaf, contributing to high surface area tension and unique water drops.

- In biology, intramolecular hydrogen bonding is partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids. The hydrogen bonds help the proteins and nucleic acids form and keep specific shapes.
Multiple-Choice Questions (MCQs) with Answers:
- What defines a hydrogen bond?
- a) Sharing of electrons
- b) Electrostatic attraction between a positively charged hydrogen atom and a highly electronegative atom
- c) Covalent bond formation
- d) Ionic bond formation
- Answer: b
- In which type of elements does hydrogen bonding primarily occur?
- a) Alkali metals
- b) Noble gases
- c) Elements with N-H, O-H, or F-H bonds
- d) Halogens
- Answer: c
- What is the primary reason for the formation of a hydrogen bond?
- a) Similar electronegativity
- b) Large atomic size
- c) Difference in electronegativity between H atom and N, O, or F atom
- d) Complete electron transfer
- Answer: c
- Which atom is commonly a hydrogen bond donor?
- a) Carbon
- b) Hydrogen
- c) Nitrogen
- d) Fluorine, oxygen, or nitrogen
- Answer: d
- What is a hydrogen bond acceptor?
- a) A hydrogen atom
- b) An atom with high electronegativity
- c) An atom with low electronegativity
- d) An atom bonded to hydrogen
- Answer: b
- What is the strength of a hydrogen bond compared to other forces?
- a) Weaker than van der Waals forces
- b) Stronger than covalent bonds
- c) Stronger than van der Waals forces, but weaker than covalent bonds
- d) Equal to ionic bonds
- Answer: c
- Which molecule demonstrates the strongest hydrogen bond due to electronegativity?
- a) Hydrogen chloride
- b) Hydrogen fluoride
- c) Hydrogen bromide
- d) Hydrogen iodide
- Answer: b
- What property contributes to the solubility of lower alcohols in water?
- a) High volatility
- b) Hydrogen bonding
- c) Low boiling point
- d) Low viscosity
- Answer: b
- Why do compounds with hydrogen bonding have higher boiling points?
- a) Weaker intermolecular forces
- b) Greater volatility
- c) Stronger hydrogen bonding
- d) Lower viscosity
- Answer: c
- In DNA, what holds the complementary strands together?
- a) Ionic bonds
- b) Covalent bonds
- c) Hydrogen bonds
- d) Metallic bonds
- Answer: c
- What contributes to the high surface tension in water?
- a) Weak van der Waals forces
- b) Strong covalent bonds
- c) Hydrogen bonding
- d) Metallic bonding
- Answer: c
- Which of the following molecules contains a hydrogen bond acceptor?
- a) Methane
- b) Diethyl ether
- c) Hydrogen sulfide
- d) Chloroform
- Answer: b
- What role does hydrogen bonding play in the viscosity of associated molecules?
- a) Decreases viscosity
- b) No effect on viscosity
- c) Increases viscosity
- d) Creates volatility
- Answer: c
- Which of the following elements commonly participates in hydrogen bonding?
- a) Carbon
- b) Hydrogen
- c) Nitrogen
- d) Chlorine
- Answer: c
- What is responsible for the unique boiling point of water?
- a) Hydrogen bonding
- b) Covalent bonding
- c) Metallic bonding
- d) Weak van der Waals forces
- Answer: a
- In proteins, what contributes to the secondary, tertiary, and quaternary structures?
- a) Covalent bonds
- b) Ionic bonds
- c) Hydrogen bonds
- d) Metallic bonds
- Answer: c
- What is the nature of the hydrogen bond in hydrogen fluoride?
- a) Weak
- b) Strong
- c) Non-existent
- d) Metallic
- Answer: b
Frequently Asked Questions (FAQs):
1. What is a hydrogen bond?
- A hydrogen bond is an electromagnetic attraction between a partially positively charged hydrogen atom and a highly electronegative atom nearby.
2. Is a hydrogen bond a true chemical bond?
- No, a hydrogen bond is a type of dipole-dipole interaction and not a true chemical bond.
3. Where can hydrogen bonds occur?
- Hydrogen bonds can occur between molecules (intermolecularly) or within various parts of a single molecule (intramolecularly).
4. What is the hydrogen bond donor?
- The hydrogen bond donor is a hydrogen atom attached to a relatively electronegative atom, such as fluorine, oxygen, or nitrogen.
5. What is a hydrogen bond acceptor?
- A hydrogen bond acceptor is an atom, whether bonded to hydrogen or not, that attracts a lone pair of electrons from another atom.
6. How strong is a hydrogen bond compared to other forces?
- The hydrogen bond is a weak bond, with strength between weak van der Waals forces and strong covalent bonds.
7. What influences the strength of a hydrogen bond?
- The strength of a hydrogen bond depends on the attraction of the shared pair of electrons, influenced by the electronegativity of the atoms involved.
8. Give an example of a strong hydrogen bond.
- Hydrogen bonding in hydrogen fluoride, where fluorine, with high electronegativity, forms the strongest hydrogen bond.
9. How does hydrogen bonding contribute to water’s properties?
- Hydrogen bonding in water leads to unique properties like a high boiling point (100 °C) and high surface tension.
10. What role does hydrogen bonding play in DNA?
- Hydrogen bonds hold the complementary strands of DNA together, contributing to the structure and stability of the molecule.
11. Why are lower alcohols soluble in water?
- Lower alcohols are soluble in water due to hydrogen bonding between water and alcohol molecules.
12. How does hydrogen bonding affect the boiling point of compounds?
- Compounds with hydrogen bonding have a higher boiling point, making them less volatile.
13. What properties are influenced by hydrogen bonding in associated molecules?
- Hydrogen bonding contributes to higher viscosity and surface tension in substances with associated molecules.
14. Give an application of hydrogen bonds in biology.
- In biology, hydrogen bonds contribute to the secondary, tertiary, and quaternary structures of proteins and nucleic acids.
15. How do hydrogen bonds contribute to high surface tension in water drops?
- Hydrogen bonds between water molecules in water drops are stronger than other intermolecular forces, leading to high surface tension.
16. What is the significance of hydrogen bonding in the summary?
- Hydrogen bonding is a crucial attractive force, forming compounds vital for life, influencing DNA structure, and contributing unique properties to water.
17. Are hydrogen bonds stronger than covalent bonds?
- No, hydrogen bonds are weaker than covalent bonds but stronger than weak van der Waals forces.
Summary:
In conclusion, hydrogen bonding plays a vital role in shaping the properties, structures, and behaviors of molecules. This electromagnetic attraction occurs between a partially positively charged hydrogen atom and a nearby highly electronegative atom, resulting in unique interactions. The tutorial covers the formation of hydrogen bonds, highlighting the donor and acceptor roles.
The strength of hydrogen bonds lies between weak van der Waals forces and strong covalent bonds. Numerous examples, such as hydrogen fluoride, water, ammonia, alcohols, and carboxylic acids, demonstrate the diverse applications of hydrogen bonding in different compounds.
The properties influenced by hydrogen bonding include solubility, volatility, viscosity, and surface tension. These effects are particularly evident in water, contributing to its distinctive features like a high boiling point and surface tension.
Hydrogen bonds are crucial in biological molecules, holding DNA strands together and contributing to the structures of proteins and nucleic acids. The summary emphasizes the significance of hydrogen bonding in forming the foundation of life and influencing the unique properties of various compounds.