What are S- Block Elements?
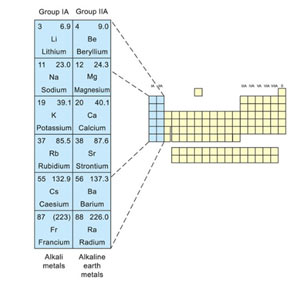
The s-block elements are the metals in Group IA and Group IIA of the periodic table.
- They are called the s-block elements because s-orbitals are being filled, in their outermost shells.
- The elements of group IA other than hydrogen are called “Alkali metals” while those of IIA are called “Alkaline-earth metals”.
- The name alkali came from Arabic, which means ‘The Ashes’.
- The Arabs used this term for these metals because they found that the ashes of plants were made chiefly of salt and potassium.
- Alkali metals consist of the elements, lithium, sodium, potassium, rubidium, caesium, and francium.
- These are very reactive metals and produce strong alkaline solutions with water.
- The alkaline-earth metals are beryllium, magnesium, calcium, strontium, barium, and radium.
- They are called alkaline-earth because they produce alkalies in water and are widely distributed in the earth’s crust.
- The alkali and alkaline-earth metals include the most reactive electropositive elements and a study of their electronic configuration will assist in understanding their properties.
Electronic Configurations of s-Block Components
Alkali Metals
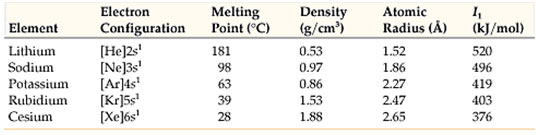
Alkali metals have only one electron in’s’ orbital of their valence shell. All alkali metals lose one electron of the valence shell to form mono-positive ions M+ because their ionization energy values are really low. They form ionic compounds and show +1 oxidation state.
Alkaline-Earth Metals
Alkaline earth metals have 2 electrons in’s’ orbital of their valence shell. All alkaline earth metals lose their two electrons to form dipositive ions M2+, because their ionization energy values are low. They form ionic substances and show + 2 oxidation state.
In going down a group, the number of shells increases by one at each step and equal to the number of the period to which the elements belong.
The occurrence of Alkali Metals
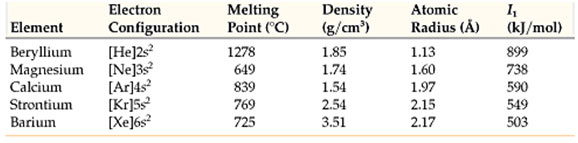
Due to high reactivity, the alkali metals are present in nature in the combined state. None of the alkali metals is discovered complimentary in nature. Sodium and potassium are abundant alkali metals and each makes up about 2.4 percent of earth’s crust. The majority of the earth’s crust is made up of insoluble alumino-silicates of alkali metals.
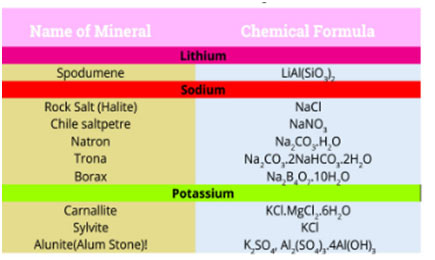
Lithium deposits, typically in the form of complicated minerals, are commonly spread over the earth. An important commercial source of lithium is the mineral spodumene, LiAl (SiO3)2. Percentages of rubidium and caesium are discovered in potassium salts deposits. Francium has actually not been discovered in nature. It has been prepared artificially in the laboratory and is very unsteady so very little is known about this metal.
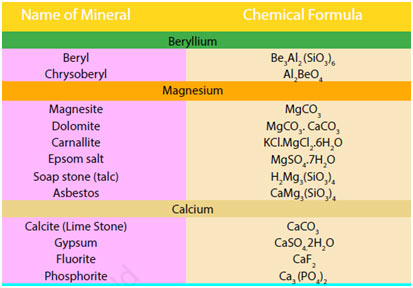
The occurrence of Alkaline-Earth Metals
Being reactive, alkaline earth metals also do not occur in a free state. The substances of these metals occur commonly in nature. Magnesium and calcium are abundant in earth’s crust. The outer portion of the earth was originally in the form of silicates and aluminosilicates of alkaline-earth metals.
Magnesium and calcium, with salt and potassium, exist in the rocks as cations. Magnesium halides are found in seawater. Magnesium is an essential constituent of chlorophyll.
Calcium phosphate, Ca3(PO4) 2, and calcium fluoride, CaF2 are also found as minerals. Calcium is a necessary constituent of numerous living organisms. It takes place as a skeletal product in bones, teeth, sea shells and eggshells. Radium is a rare element. It is of great interest because of its radioactive nature.
Peculiar Behaviour of Lithium
In a number of its properties, lithium is rather different from other alkali metals. This behaviour is not unusual, because the very first member of each main group of the periodic table reveals marked deviation from the regular patterns of the group as a whole.
The deviation revealed by lithium can be explained based on its small radius and high charge density. The nuclear charge of Li+ ion is screened only by a shell of 2 electrons. The so-called ‘anomalous’ properties of lithium are because lithium is unexpectedly far less electropositive than sodium. A few of the more crucial distinctions of lithium from other alkali metals are listed below:
- Lithium is much harder and lighter than other alkali metals.
- The lithium salts of anions with high charge density are normally less soluble in water than those of the other alkali metals, e.g. LiOH, LiF, Li3PO4, Li2CO3.
- Lithium forms steady complex substances, although complex development normally is not a property of alkali metals. One of the stable complexes formed by lithium is [Li (NH3) 4] +.
- Lithium reacts extremely slowly with water, while other alkali metals respond strongly.
- Lithium salts of big polarizable anions are less stable than those of other alkali metals. Unlike other alkali metals, lithium does not form bicarbonate, tri-iodide or hydrogen sulphide at room temperature.
- When burnt in air lithium forms just typical oxide, whereas the others form peroxides or superoxides.
- Lithium hydride is steadier than the hydrides of other alkali metals.
- Lithium substances are more covalent, which is why its halides are more soluble in natural solvents and the alkyls and aryls of lithium are more stable than those of other alkali metals.
- Lithium is the least reactive metal of all the alkali metals.
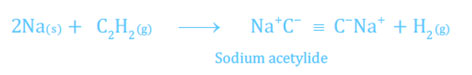
- When acetylene is passed over highly heated up lithium, it does not produce lithium acetylide, but other alkali metals form the matching metallic acetylides.
- Lithium has low electropositive character; thus, its carbonate and nitrate are not so stable and for that reason decomposition giving lithium oxide. Carbonates of other alkali metals do not decompose. The decomposition of lithium nitrate offers different products than the nitrates of other alkali metals.
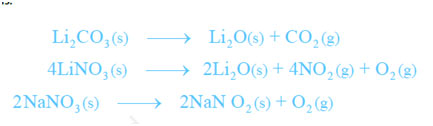
- Lithium chloride has an exothermic heat of solution, whereas chlorides of salt and potassium have endothermic heats up of the solution.
- Lithium carbide is the only alkali metal carbide formed easily by the direct reaction.
- Lithium hydroxide when highly heated up produces lithium oxide however the other alkali metal hydroxides do not show this behaviour.
![]()
- Lithium reacts with nitrogen to form nitride, while the other members of the group do not give this reaction.

Peculiar Behaviour of Beryllium
Beryllium is the lightest member of the series and differs from the other group IIA elements in numerous ways. This is due to its small atomic size and relatively high electronegativity value. The bottom lines of difference are:
- Beryllium metal is almost as hard as iron and hard to scratch glass. The other alkaline earth metals are much softer than beryllium but still harder than the alkali metals.
- The melting and boiling points of beryllium are higher than other alkaline earth metals.
- As reducing agents, the group IIA metals are all-powerful and adequate to minimize water, at least in principle. Nevertheless, with water, beryllium forms insoluble oxide coating that secures it from additional attack.
- Beryllium, in particular, is quite resistant to complete oxidation, even by acids, because of its BeO coating.
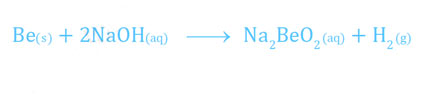
- Beryllium is the only member of its group which reacts with alkalies to form hydrogen. The other members do not react with alkalies.
MCQs
- What is the primary reason for calling these elements “s-Block Elements”?
- a) They have s-shaped electronic configurations
- b) Their outermost shells are filled with s-orbitals
- c) They have a specific s-block in the periodic table
- d) They have a special kind of s-bonding
- Answer: b
- What are the elements in Group IA of the periodic table called?
- a) Alkaline-earth metals
- b) Transition metals
- c) Alkali metals
- d) Halogens
- Answer: c
- Which metals are referred to as “Alkaline-earth metals” in the s-Block?
- a) Group IA elements
- b) Transition metals
- c) Group IIA elements
- d) Noble gases
- Answer: c
- How many electrons do alkali metals have in the ‘s’ orbital of their valence shell?
- a) One
- b) Two
- c) Three
- d) Four
- Answer: a
- What is the oxidation state of alkali metals when they form ions?
- a) +1
- b) -1
- c) +2
- d) -2
- Answer: a
- What is the electronic configuration of alkaline-earth metals?
- a) s²
- b) s²p³
- c) s¹²
- d) s²²
- Answer: a
- Why are alkali metals rarely found in a free state in nature?
- a) They are highly reactive
- b) They form strong alkaline solutions
- c) They have low ionization energy
- d) They readily combine with oxygen
- Answer: a
- Which mineral is mentioned as an important commercial source of lithium?
- a) Salt
- b) Bauxite
- c) Spodumene
- d) Quartz
- Answer: c
- What is the oxidation state of alkaline earth metals when they form ions?
- a) +1
- b) -1
- c) +2
- d) -2
- Answer: c
- Why does lithium exhibit peculiar behavior compared to other alkali metals?
- a) It has a larger atomic radius
- b) It has a smaller atomic radius
- c) It has a higher charge density
- d) It has a lower charge density
- Answer: c
- What is one of the stable complexes formed by lithium?
- a) [Li(OH)₂]
- b) [Li(NH₃)₄]⁺
- c) [LiF]₂
- d) [Li₂CO₃]₃
- Answer: b
- How does lithium react with water compared to other alkali metals?
- a) Reacts rapidly
- b) Reacts moderately
- c) Reacts slowly
- d) Does not react
- Answer: c
- What is the key difference in the behavior of lithium chloride’s heat of solution compared to other alkali metals’ chlorides?
- a) Endothermic
- b) Exothermic
- c) No heat is released
- d) No heat is absorbed
- Answer: b
- Which metal in the s-Block reacts with alkalies to form hydrogen?
- a) Sodium
- b) Lithium
- c) Potassium
- d) Beryllium
- Answer: d
- Which of the following is a peculiar behavior of lithium compared to other alkali metals?
- a) Rapid reaction with water
- b) Formation of bicarbonates
- c) Formation of stable complex substances
- d) Reactivity with nitrogen
- Answer: c
- What property of beryllium makes it distinct from other alkaline earth metals?
- a) High electronegativity
- b) Low melting and boiling points
- c) Formation of insoluble oxide coating with water
- d) Resistance towards oxidation by acids
- Answer: c
- Which of the following is a characteristic of beryllium carbide compared to other alkali metal carbides?
- a) Easily formed by direct reaction
- b) Highly unstable
- c) Insoluble in organic solvents
- d) Doesn’t react with alkalies
- Answer: a
- What makes beryllium resistant towards complete oxidation by acids?
- a) High electronegativity
- b) Formation of an oxide coating
- c) Low melting point
- d) Reactivity with alkalies
- Answer: b
- Which alkali metal does not form bicarbonates, tri-iodide, or hydrogen sulfide at room temperature?
- a) Lithium
- b) Sodium
- c) Potassium
- d) Rubidium
- Answer: a
- What happens when acetylene is passed over highly heated up lithium compared to other alkali metals?
- a) Forms metallic acetylides
- b) Does not react
- c) Forms stable complexes
- d) Releases hydrogen gas
- Answer: b
- What does the decomposition of lithium nitrate result in?
- a) Lithium oxide and nitrogen
- b) Lithium peroxide
- c) Lithium carbonate
- d) Lithium hydroxide
- Answer: a
- Which of the following has a higher heat of solution: lithium chloride or chlorides of salt and potassium?
- a) Lithium chloride
- b) Chlorides of salt and potassium
- c) Both have similar heat of solution
- d) None of the above
- Answer: a
- What is the primary reason for the resistance of beryllium towards complete oxidation?
- a) High electronegativity
- b) Formation of an oxide coating
- c) Low melting and boiling points
- d) Reactivity with alkalies
- Answer: b
Frequently Asked Questions (FAQs)
- What are S-Block Elements?
- S-Block elements are metals located in Group IA and Group IIA of the periodic table. They are named so because the s-orbitals in their outermost shells are being filled.
- How are Alkali Metals different from Alkaline-Earth Metals?
- Alkali metals are in Group IA and include lithium, sodium, potassium, rubidium, caesium, and francium. Alkaline-Earth metals are in Group IIA and include beryllium, magnesium, calcium, strontium, barium, and radium.
- What is the origin of the term “alkali” in Alkali Metals?
- The term “alkali” comes from Arabic, meaning ‘The Ashes.’ It was used because the Arabs found that plant ashes were primarily composed of salt and potassium.
- How reactive are Alkali Metals and Alkaline-Earth Metals?
- These metals are highly reactive, producing strong alkaline solutions when in contact with water.
- What is the occurrence of Alkali Metals in nature?
- Due to their high reactivity, alkali metals are found in nature in the combined state. None of them is found freely in nature, with sodium and potassium being abundant, making up about 2.4% of the Earth’s crust.
- How does Lithium differ from other alkali metals?
- Lithium exhibits several peculiar behaviors, such as being harder and lighter, reacting slowly with water, forming stable complex substances, and having lower reactivity compared to other alkali metals.
- Why is Beryllium unique among the Alkaline-Earth Metals?
- Beryllium differs from other alkaline-earth metals due to its hardness, higher melting and boiling points, resistance towards complete oxidation by acids, and its ability to react with alkalies to form hydrogen.
- Why does Lithium exhibit anomalous properties compared to other alkali metals?
- The anomalous properties of lithium are attributed to its small atomic radius, high charge density, and the screening of its nuclear charge only by a shell of 2 electrons.
- How does the occurrence of Alkaline-Earth Metals manifest in nature?
- Alkaline-earth metals, being reactive, do not occur freely. They are commonly found in the Earth’s crust in the form of silicates, aluminosilicates, and various minerals like magnesium halides, calcium phosphate, and calcium fluoride.
- Why is Beryllium resistant to complete oxidation, even by acids?
- Beryllium forms an insoluble oxide coating with water, which protects it from further oxidation, making it resistant even to acids.
Q11: Why alkali metals are called alkali?
Ans: The name “alkali” came from Arabic which means “The ashes”. These metals are called so because firstly they were found in ashes of plants and chiefly composed of sodium and potassium.
Q12: Why lime water turns milky with CO2?
Ans: When CO2 is passed through lime water, calcium carbonate is formed which is insoluble in water, due to which lime water turns milky.
Q13: When does milky lime water become clear?
Ans: When excess CO2 is passed through milky lime water, soluble calcium carbonate is formed. Thus, the solution becomes clear.
Q14: What are the properties which make beryllium unique within its own family?
- Beryllium is as hard as iron.
- It has high melting and boiling points than other alkaline metals.
- It is the only member of family which reacts with alkalies to give hydrogen.
Summary
The tutorial on S-Block Elements and Peculiar Behavior of their Representatives explains the fundamental characteristics of alkali metals, alkaline-earth metals, and their anomalous behaviors, focusing on lithium and beryllium.
Key Points:
- S-Block Elements:
- Metals in Group IA and Group IIA of the periodic table are termed S-Block Elements.
- Alkali metals (Group IA) and alkaline-earth metals (Group IIA) are highly reactive electropositive elements.
- Occurrence:
- Alkali metals, including lithium, sodium, potassium, etc., are found in nature in combined states due to their high reactivity.
- Alkaline-earth metals like magnesium and calcium occur abundantly in the Earth’s crust as silicates and aluminosilicates.
- Electronic Configurations:
- Alkali metals have a single electron in the ‘s’ orbital, leading to the formation of mono-positive ions (M+).
- Alkaline-earth metals have two electrons in the ‘s’ orbital, resulting in dipositive ions (M2+).
- Peculiar Behavior of Lithium:
- Lithium exhibits unique properties due to its small radius and high charge density.
- It reacts slowly with water, forms stable complexes, and its salts have lower solubility compared to other alkali metals.
- Peculiar Behavior of Beryllium:
- Beryllium, the lightest in Group IIA, differs from other members due to its hardness and resistance to oxidation.
- It reacts with alkalies to form hydrogen, setting it apart from other alkaline-earth metals.
The tutorial provides a comprehensive understanding of these elements, their electronic configurations, occurrences in nature, and the intriguing behaviors that distinguish them from one another. If you have further questions, refer to the FAQs section for additional insights.