Periodic Table
In the nineteenth century, chemists committed much of their efforts in attempts to set up elements in a periodic manner.
Those who made memorable contributions in this field are Al-Razi, Dobereiner, Newland, and Mendeleev.
Al-Razi’s classifications were based upon the physical and chemical properties of elements.
Dobereiner, a German chemist in 1829, arranged the identified elements in a group called Triads, as each consisted of 3 elements with similar properties. Newland who was an English chemist, in 1864, categorized 62 elements, known at that time, in increasing order of their atomic masses.
He noticed that every eighth component had some properties in common with the very first one. The concept on which this classification is based is called the Law of octaves.
Dobereiner’s Triads
A German chemist Dobereiner observed relationship between atomic masses of several groups of three elements called triads. In these groups, the main or middle component had an atomic mass average of the other two components.
One triad group example is that of calcium (40), strontium (88) and barium (137). The atomic mass of strontium is the average of the atomic masses of calcium and barium. Just a couple of components could be set up in this way. This category did not get wide acceptance.
Newlands Octaves
After a successful decision of appropriate atomic masses of components by Cannizzaro in 1860, efforts were again started to organize components. In 1864 British chemistNewlands put forward his observations in the form of ‘law of octaves’.
He kept in mind that there was a repetition in chemical properties of every 8th component if they were set up by their increasing atomic masses. He compared it with musical notes. His work could not get much recognition as no space was left for undiscovered elements. The noble gases were also not known at that time.
Mendeleev’s Periodic Table
In 1871, a Russian chemist, Dmitri Mendeleev, offered a better and detailed plan for the classification of components. He provided the first regular table of elements in which elements of similar chemical properties were set up in 8 vertical columns called Groups.
The horizontal rows of the table were called Periods. Mendeleev set up the known elements (just 63) in order of increasing atomic masses, in horizontal rows called periods. So that components with similar properties remained in the exact same vertical columns. This arrangement of elements was called Periodic Table.
He put forward the results of his work in the form of the periodic law, which is stated as:
“properties of the elements are periodic functions of their atomic masses”.
Demerits of Mendeleev’s Periodic Table
Although, Mendeleev periodic table was the very first attempt to organize the elements, yet it has a couple of demerits in it. His failure to discuss the position of isotopes and wrong order of the atomic masses of some elements recommended that atomic mass of an element can not serve as the basis for the arrangement of components.
Improvements in Mendeleev’s Periodic Table
In order to make the periodic table more useful and precise, a few improvements were made in Mendeleev s periodic table. In1913 H. Moseley discovered a new property of the components i.e. an atomic number. He observed that atomic number instead of atomic mass must identify the position of the component in the periodic table and accordingly the periodic law was amended as:
” properties of the elements are periodic function of their atomic numbers”.
An atomic number of an element equals the number of electrons in a neutral atom. So atomic number provides the basis of electronic configuration as well.
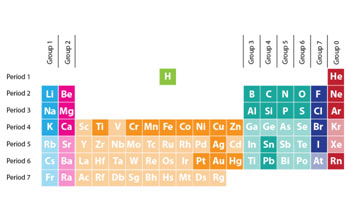
The Modern Periodic Table:
In the modern periodic table, all the elements are arranged in increasing order of their atomic numbers. Following are the essential features of the periodic table.

-
Group and Periods
Elements with similar properties are placed in vertical columns called Groups. There are 8 groups, which are normally numbered by Roman characters I to VIII. Each group is divided into two subgroups, designated as A and B subgroups. The subgroups, including the representative or normal elements, are identified as A subgroups, whereas B subgroup consists of less typical elements, called transition elements and are organized in the centre of the periodic table of elements. The horizontal rows of the table of elements are called Periods. The essential features of the periods are as follows:
- a) There are 7 periods in the table of elements numbered by Arabic characters 1 to 7.
- b) The period 1 includes only two elements, hydrogen and helium.
- c) The period 2 and 3 include 8 elements each and are called short periods. All the elements in these periods are representative elements and belong to A subgroup. In these periods, every 8th component resembles in properties with the first component. As lithium and beryllium in the second period look like in the majority of their properties with sodium and magnesium of the 3rd period, respectively. Similarly, boron and aluminium both show oxidation state of +3, fluorine in 2nd period have close similarities with chlorine of the 3rd period.
- d) The periods 4 and 5 are called long periods. Each long period includes eighteen elements. Out of these, eight are representative elements coming from A subgroup comparable to 2nd and third periods. Whereas the other 10 elements, positioned in the centre of the table come from B subgroups and are called transition elements. In these periods, the repetition of properties amongst the elements happens after 18 elements. As after 19K (having atomic number 19) the next element with similar properties is 37
- e) The 6th period is also a long period, which contains thirty-two elements. In this duration there are 8 representative elements, 10 transition elements and a new set of fourteen elements called Lanthanides as they begin after 57 Lanthanides have remarkably similar properties and are normally shown individually at the bottom of the periodic table.
- f) The 7th period is incomplete so far. It includes only two typical elements 87Fr and 88Ra, ten transition elements and fourteen inner transition elements. The inner transition elements of this period are called Actinides, as they follow 89 The actinides are also shown at the bottom of the periodic table of elements under the Lanthanides. Due to their scarcity, the inner transition elements are also called rare earth elements.
-
Some More Families in the Periodic Table:
While studying about periods you have noticed that certain rows of elements with similar properties have actually appointed typical names such as transition elements, Lanthanides, Actinides or Rate Earth elements.
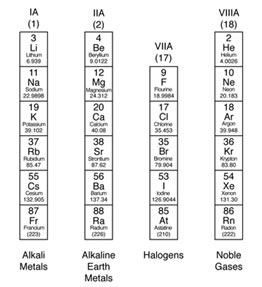
Similarly, due to their peculiar attributes, some common elements coming from sub-groups A, have actually also been assigned family names. For example, elements of the group IA are called Alkali Metals, because of their property to form strong alkalies with water.
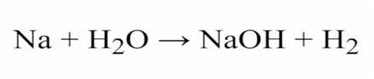
Likewise, due to their existence in Earth’s crust and alkaline character, the elements of group IIA are referred to as Alkaline Earth Metals. Another essential family in the table of elements is Halogen family.
The name “Halogens” is provided to the components of group VIIA, due to their salt-forming properties. As the gases of group VIIIA ‘are least reactive they are called “Noble Gases”. These family names work for a fast acknowledgement of an element in the periodic table.
-
Blocks in the Periodic Table
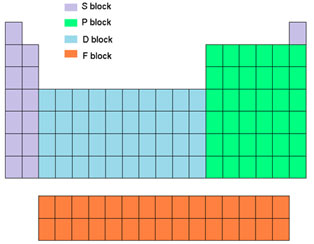
Elements in the periodic table can also be classified into 4 blocks. This classification is based upon the valence orbital of the elements involved in chemical bonding. According to this category, elements of IA and IIA subgroups are called s-block elements because their valence electrons are readily available in s orbital. The elements of IIIA to VlllA subgroups (other than He) are called p-block components as their valence electrons exist in the p orbital.
Similarly, in transition elements, electrons in d-orbital are responsible for their valency thus they are called d-block elements. For Lanthanides and Actinides valence electrons exist in f- orbital hence these elements are called f-block elements. This category is quite useful in understanding the chemistry of elements and predicting their properties especially the principle of valency or oxidation state.
-
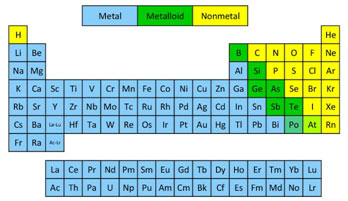
Metals, Non-metals and Metalloids
Another basis for classifying the elements in the periodic table is their metal character. Generally, the components on the left-hand side, in the centre and at the bottom of the periodic table are metals, while the non-metals remain in the upper right corner of the table.
Some elements, specifically lower members of groups, IIIA, IVA and VA have properties of both metals as well as non-metals. These elements are called semi-metals or metalloids. In the periodic table elements of groups IVA to VIIIA, at the top right-hand corner above the stepped line, are non-metals. The elements simply under the “steps’ such as Si, As, and Te are the metalloids. All the remaining elements, other than hydrogen, are metals.
MCQs with Answers: Periodic Table of Elements
- What is the Periodic Table based on?
- A) Atomic Numbers
- B) Atomic Masses
- C) Number of Protons
- D) Number of Neutrons
Answer: A) Atomic Numbers
- Who proposed the concept of Triads in the classification of elements?
- A) Al-Razi
- B) Dobereiner
- C) Newlands
- D) Mendeleev
Answer: B) Dobereiner
- Which law is associated with Newlands’ classification of elements?
- A) Law of Triads
- B) Law of Octaves
- C) Law of Elements
- D) Law of Periods
Answer: B) Law of Octaves
- Who presented the first regular table of elements in 1871?
- A) Al-Razi
- B) Dobereiner
- C) Newlands
- D) Mendeleev
Answer: D) Mendeleev
- What did Mendeleev arrange in vertical columns in his Periodic Table?
- A) Elements with similar properties
- B) Elements with the same atomic mass
- C) Elements with the same atomic number
- D) Elements with the same number of electrons
Answer: A) Elements with similar properties
- What did Mendeleev’s Periodic Table fail to explain?
- A) Isotopes
- B) Atomic Numbers
- C) Chemical Properties
- D) Transition Elements
Answer: A) Isotopes
- What property did Moseley discover that improved the Periodic Table?
- A) Atomic Mass
- B) Atomic Number
- C) Atomic Radius
- D) Atomic Weight
Answer: B) Atomic Number
- How are elements arranged in the Modern Periodic Table?
- A) Increasing Atomic Mass
- B) Increasing Atomic Number
- C) Decreasing Atomic Radius
- D) Decreasing Ionization Energy
Answer: B) Increasing Atomic Number
- How many groups are there in the Modern Periodic Table?
- A) 6
- B) 7
- C) 8
- D) 9
Answer: C) 8
- What are elements with similar properties placed in vertical columns called?
- A) Rows
- B) Periods
- C) Groups
- D) Blocks
Answer: C) Groups
- Which family in the Periodic Table is known for forming strong alkalies with water?
- A) Halogens
- B) Alkali Metals
- C) Noble Gases
- D) Alkaline Earth Metals
Answer: B) Alkali Metals
- What is another name for the elements of Group IIA in the Periodic Table?
- A) Noble Gases
- B) Alkali Metals
- C) Alkaline Earth Metals
- D) Halogens
Answer: C) Alkaline Earth Metals
- Which block in the Periodic Table includes elements with valence electrons in the s orbital?
- A) s-block
- B) p-block
- C) d-block
- D) f-block
Answer: A) s-block
- Where are the metalloids located in the Periodic Table?
- A) Left side
- B) Right side
- C) In the center
- D) At the top
Answer: C) In the center
- Which elements are generally found on the left-hand side, in the center, and at the bottom of the Periodic Table?
- A) Alkali Metals
- B) Noble Gases
- C) Transition Elements
- D) Metals
Answer: D) Metals
- Which element is considered a semi-metal or metalloid?
- A) Silicon (Si)
- B) Sodium (Na)
- C) Iron (Fe)
- D) Fluorine (F)
Answer: A) Silicon (Si)
- How many periods are there in the Periodic Table?
- A) 6
- B) 7
- C) 8
- D) 9
Answer: B) 7
- What is the characteristic feature of long periods in the Periodic Table?
- A) 8 representative elements
- B) 18 representative elements
- C) 32 representative elements
- D) 16 representative elements
Answer: C) 32 representative elements
- What is the name given to the elements of Group VIIA due to their salt-forming properties?
- A) Noble Gases
- B) Alkali Metals
- C) Halogens
- D) Alkaline Earth Metals
Answer: C) Halogens
- Which block in the Periodic Table includes elements with valence electrons in the p orbital?
- A) s-block
- B) p-block
- C) d-block
- D) f-block
Answer: B) p-block
- Where are non-metals generally located in the Periodic Table?
- A) Bottom right corner
- B) Top left corner
- C) Bottom left corner
- D) Top right corner
Answer: A) Bottom right corner
Frequently Asked Questions (FAQs): Periodic Table of Elements
- What is the Periodic Table based on?
- The Periodic Table is based on the increasing order of atomic numbers.
- Who proposed the concept of Triads in the classification of elements?
- The concept of Triads was proposed by the German chemist Dobereiner.
- What is the Law of Octaves?
- The Law of Octaves, proposed by Newlands, states that there is a repetition in chemical properties of every 8th element when arranged by increasing atomic masses.
- Who presented the first regular table of elements in 1871?
- Dmitri Mendeleev, a Russian chemist, presented the first regular table of elements.
- What is Mendeleev’s Periodic Law?
- Mendeleev’s Periodic Law states that the properties of elements are periodic functions of their atomic masses.
- What are the demerits of Mendeleev’s Periodic Table?
- Mendeleev’s Periodic Table failed to explain the position of isotopes and had errors in the order of atomic masses for some elements.
- How was Mendeleev’s Periodic Table improved?
- The improvement came with the discovery of the atomic number by H. Moseley in 1913, leading to the amendment of the Periodic Law as properties being a periodic function of atomic numbers.
- How are elements arranged in the Modern Periodic Table?
- Elements in the Modern Periodic Table are arranged in increasing order of their atomic numbers.
- What are the essential features of the Periodic Table?
- The Periodic Table includes Groups (vertical columns) and Periods (horizontal rows), where elements with similar properties are placed in groups.
- How many groups are there in the Modern Periodic Table?
- There are 8 groups in the Modern Periodic Table, numbered I to VIII.
- What are the characteristics of short and long periods in the Periodic Table?
- Short periods (periods 2 and 3) have 8 representative elements each, while long periods (periods 4, 5, and 6) include 18 representative elements and transition elements.
- What are the families in the Periodic Table?
- Families in the Periodic Table include Alkali Metals, Alkaline Earth Metals, Halogens, and Noble Gases, named based on their properties.
- How are elements classified into blocks in the Periodic Table?
- Elements are classified into s-block, p-block, d-block, and f-block based on the valence orbital involved in chemical bonding.
- What is the basis for classifying elements as metals, non-metals, and metalloids?
- Elements on the left, center, and bottom of the Periodic Table are metals, while non-metals are in the upper right corner. Metalloids have properties of both metals and non-metals.
- Where are metalloids located in the Periodic Table?
- Metalloids are located in the center of the Periodic Table, just below the “steps.”
- What are the semi-metals in the Periodic Table?
- Semi-metals, or metalloids, include lower members of groups IIIA, IVA, and VA, such as Silicon (Si), which exhibit properties of both metals and non-metals.
- How many periods are there in the Periodic Table?
- There are 7 periods in the Periodic Table, numbered 1 to 7.
- What are Lanthanides and Actinides?
- Lanthanides and Actinides are inner transition elements, with Lanthanides starting after element 57 and Actinides following element 89.
- Why are inner transition elements also called rare earth elements?
- Inner transition elements, or rare earth elements, are called so due to their scarcity and are shown at the bottom of the Periodic Table.
- Which elements are considered Noble Gases?
- Noble Gases are the elements in Group VIIIA, known for their least reactivity.
- What does the classification of elements into blocks help predict?
- The classification into blocks helps predict the chemistry of elements, especially in terms of valency or oxidation state.
Q22: What is periodicity?
Ans: The recapitulation or repetition of properties of elements in the periodic table after regular intervals is called periodicity.
Q23: Which element has the highest electronegativity?
Ans: Fluorine, the halogen has the highest electronegativity value of 4.
Q24: Which is the longest period in the periodic table?
Ans: The 6th and 7th periods are the longest with 32 inner transition elements each.
Q25: What is the first element of the lanthanide series?
Ans: Lanthanum is the first one.
Q26: Dissimilarities between Hydrogen and IA group of the periodic table?
| Hydrogen | IA Group |
| Non-metal | Metals |
| Accepts electron. | Do not gain electrons. |
| Gas at room temperature. | Solid at room temperature. |
Summary: Periodic Table of Elements Tutorial
In this comprehensive tutorial on the Periodic Table of Elements, we explored the historical developments and contributions of chemists such as Al-Razi, Dobereiner, Newland, and Mendeleev. The tutorial covers the early attempts at classification through Dobereiner’s Triads and Newlands’ Law of Octaves. Mendeleev’s groundbreaking work in 1871 presented the first regular table, organizing elements by similar chemical properties and atomic masses.
The demerits of Mendeleev’s table, including its inability to explain isotopes and incorrect atomic mass orders, led to improvements. The discovery of atomic number by H. Moseley in 1913 became pivotal, prompting the amendment of the Periodic Law to state that properties are periodic functions of atomic numbers.
The Modern Periodic Table, based on increasing atomic numbers, is examined with a focus on groups, periods, and families. The tutorial delves into the classification of elements into s-block, p-block, d-block, and f-block based on valence orbitals. The distinction between metals, non-metals, and metalloids is explored, along with the categorization of elements into specific families like Alkali Metals, Alkaline Earth Metals, Halogens, and Noble Gases.
By understanding the periodic table’s structure and organization, including blocks and families, learners gain insights into predicting elemental properties and behaviors. The tutorial serves as a valuable resource for those seeking a comprehensive understanding of the Periodic Table of Elements.
