Measurement of e/m Value of Electron and Mass of Electron
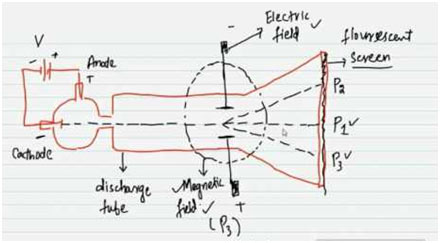
In 1897, J.J Thomson devised an instrument to measure the e/m value of electrons. The device includes a discharge tube.
- The cathode rays are permitted to go through electric and magnetic fields.
- When both the fields are off then a beam of cathode rays, including electrons, produces a luminous area at P1 on the fluorescent screen.
- The north and south poles of the magnetic field are perpendicular to the plane of the paper in the diagram.
- The electrical field is in the plane of the paper. When the only magnetic field is used, the cathode rays are deflected in a circular path and fall at point P3.
- When only the electrical field is used, the cathode rays produce an area at P2.
- Both electrical and magnetic fields are then used at the same time and their strengths change in such a way that cathode rays once again strike the point P1.
- In this way, by comparing the strengths of the two fields one can identify the e/m value of electrons. It comes out to be 1.7588 x 1011 coulombs kg-1. This indicates that 1 kg of electrons has 1.7588 x 1011 coulombs of charge.
Measurement of Charge on Electron – Millikan’s Oil Drop Experiment
The oil drop experiment was carried out by Robert A. Millikan and Harvey Fletcher in 1909 to determine the elementary electrical charge. The experiment happened in the Ryerson Physical Laboratory at the University of Chicago. Millikan received the Nobel Reward in Physics in 1923.
In 1909, Millikan identified the charge on an electron by a basic arrangement. The apparatus includes a metallic chamber. It has two parts. The chamber is filled with air, the pressure of which can be changed by a vacuum pump. There are two electrodes A and A’. These electrodes are used to create an electrical field in the area in between the electrodes. The upper electrode has a hole in it.

A fine spray of oil droplets is created by an atomizer. A couple of droplets travels through the hole in the top plate and into the area between the charged plates, where one of them is observed through a microscope. This droplet, when brightened perpendicularly to the direction of view, appears in the microscope as a bright speck against a dark background. The droplet falls under the force of gravity without applying the electric field. The speed of the droplet is figured out. The velocity of the droplet (V1) depends upon its weight, mg.
v1α mg (Eq 1)
where ‘m’ is the mass of the droplet and ‘g’ is the velocity due to gravity. After that, the air in between the electrodes is ionized by X-rays. The droplet under observation uses up an electron and gets charged. Now, connect A and A’ to a battery that produces an electric field having a strength, E. The droplet moves upwards against the action of gravity with a speed (v2).
v α Ee − mg (Eq 2)
where ‘e’ is the charge on the electron and Ee is the upward driving force on the droplet due to the applied electric field of strength E.
Dividing formula (1) by (2)
v1/v2 = mg/Ee -mg (Eq 3)
The values of v1 and v2 are recorded with the help of a microscopic lens. The elements like g and E are also understood. Mass of the droplet can be identified by differing the electrical field in such a way that the droplet is suspended in the chamber.
For this reason, ‘e’ can be computed. By changing the strength of the electrical field, Millikan discovered that the charge on each droplet was different. The tiniest charge which he found was 1.59 x 10-19 coulombs, which is very close to the recent value of 1.6022 x 10-19 coulombs.
This smallest charge on any droplet is the charge of one electron. The other drops having more than one electron on them, have double or triple the quantity of this charge. The charge present on an electron is the tiniest charge of electrical energy that has been measured so far.
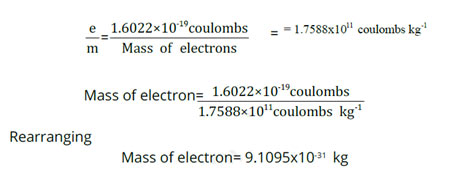
Mass of Electron
The value of charge on electron is 1.602 x 10-19 coulombs, while e/m of electron is 1.7588 x 1011 coulombs kg-1. So,
Characteristic of Fundamental Particles
The properties of 3 fundamental particles electron, proton, and neutron present in an atom are as follows.

MCQs
- 1. What did J.J. Thomson use to measure the e/m value of the electron in 1897?
- a) Cathode tube
- b) Laser beam
- c) Discharge tube
- d) X-ray machine
- Answer: c) Discharge tube
- 2. In the measurement of e/m value of the electron, what happens when only the magnetic field is applied?
- a) Cathode rays produce an area at P2
- b) Cathode rays are deflected in a circular path and fall at P3
- c) Cathode rays produce a luminous area at P1
- d) No change in the behavior of cathode rays
- Answer: b) Cathode rays are deflected in a circular path and fall at P3
- 3. How did Millikan determine the charge on an electron in his oil drop experiment?
- a) By using a vacuum pump
- b) By observing oil droplets through a microscope
- c) By releasing X-rays
- d) By changing the strength of the electrical field
- Answer: b) By observing oil droplets through a microscope
- 4. What is the charge on the electron in Millikan’s oil drop experiment?
- a) 1.7588 x 10^11 coulombs
- b) 1.6022 x 10^-19 coulombs
- c) 1.59 x 10^-19 coulombs
- d) 1.602 x 10^-19 coulombs
- Answer: c) 1.59 x 10^-19 coulombs
- 5. How did Millikan find the charge on an individual droplet in his experiment?
- a) By applying a magnetic field
- b) By releasing X-rays
- c) By changing the strength of the electrical field
- d) By observing through a telescope
- Answer: c) By changing the strength of the electrical field
- 6. What is the value of e/m for the electron according to J.J. Thomson’s measurements?
- a) 1.7588 x 10^11 coulombs/kg
- b) 1.6022 x 10^-19 coulombs
- c) 1.59 x 10^-19 coulombs
- d) 1.602 x 10^-19 coulombs
- Answer: a) 1.7588 x 10^11 coulombs/kg
- 7. What does the smallest charge on any droplet represent in Millikan’s oil drop experiment?
- a) Charge on an electron
- b) Charge on a proton
- c) Charge on a neutron
- d) Charge on a nucleus
- Answer: a) Charge on an electron
- 8. What is the mass of the electron in terms of its charge and e/m ratio?
- a) 1.7588 x 10^11 kg
- b) 9.11 x 10^-31 kg
- c) 1.6022 x 10^-19 kg
- d) 1.59 x 10^-19 kg
- Answer: b) 9.11 x 10^-31 kg
- 9. What experiment did Millikan and Fletcher carry out in 1909?
- a) Laser experiment
- b) Oil drop experiment
- c) Discharge tube experiment
- d) X-ray experiment
- Answer: b) Oil drop experiment
- 10. How did Millikan identify the charge on an electron in his oil drop experiment?
- a) By using a vacuum pump
- b) By observing oil droplets through a microscope
- c) By releasing X-rays
- d) By changing the strength of the electrical field
- Answer: b) By observing oil droplets through a microscope
- 11. What does the charge on an electron represent in Millikan’s experiment?
- a) Largest charge measured
- b) Smallest charge measured
- c) Average charge measured
- d) Charge on a proton
- Answer: b) Smallest charge measured
- 12. What is the charge on an electron in Coulombs?
- a) 1.7588 x 10^11 C
- b) 9.11 x 10^-31 C
- c) 1.6022 x 10^-19 C
- d) 1.59 x 10^-19 C
- Answer: c) 1.6022 x 10^-19 C
- 13. What does the e/m value represent in J.J. Thomson’s experiment?
- a) Mass of the electron
- b) Charge of the electron
- c) Velocity of the electron
- d) Kinetic energy of the electron
- Answer: b) Charge of the electron
FAQs – Measurement of e/m Value of Electron and Mass of Electron
1. What is the purpose of J.J. Thomson’s instrument in measuring the e/m value of the electron?
- J.J. Thomson’s instrument was designed to measure the e/m value of the electron using a discharge tube, electric and magnetic fields.
2. How did J.J. Thomson determine the e/m value of the electron in his experiment?
- By applying both electric and magnetic fields and adjusting their strengths until cathode rays struck a specific point, the e/m value of electrons was determined.
3. What is the significance of the e/m value obtained by J.J. Thomson?
- The obtained e/m value (1.7588 x 10^11 C/kg) indicates that 1 kg of electrons has 1.7588 x 10^11 coulombs of charge.
4. Who conducted the oil drop experiment, and what was its purpose?
- The oil drop experiment was conducted by Robert A. Millikan and Harvey Fletcher in 1909. Its purpose was to determine the elementary electrical charge.
5. How did Millikan determine the charge on an electron in the oil drop experiment?
- Millikan determined the charge on an electron by observing oil droplets through a microscope and adjusting the electrical field strength.
6. What was the smallest charge discovered by Millikan, and what does it represent?
- The smallest charge discovered was 1.59 x 10^-19 coulombs, representing the charge of one electron, the smallest unit of electrical energy measured.
7. What is the mass of the electron, and how is it related to its charge and e/m value?
- The mass of the electron is approximately 9.11 x 10^-31 kg. Its charge (1.602 x 10^-19 C) and e/m value (1.7588 x 10^11 C/kg) contribute to determining its mass.
8. What fundamental particles are discussed in the tutorial, and what are their characteristics?
- The tutorial discusses electrons, protons, and neutrons as fundamental particles, each with distinct properties within an atom.
Summary
This tutorial covers the methods employed to measure the e/m value of electrons and determine the mass of electrons. J.J. Thomson’s instrument, utilizing a discharge tube and electric and magnetic fields, allowed the calculation of the e/m value, yielding a significant result of 1.7588 x 10^11 coulombs kg^-1.
The tutorial then explores Millikan’s Oil Drop Experiment, conducted in 1909, which aimed to identify the elementary electrical charge. By observing oil droplets and applying an electric field, Millikan determined the charge on an electron to be 1.6022 x 10^-19 coulombs, the smallest unit of electrical energy measured.
The mass of the electron is related to its charge and e/m value, with an approximate value of 9.11 x 10^-31 kg. The tutorial concludes by outlining the characteristics of fundamental particles—electrons, protons, and neutrons—present in an atom.
