Definition
Western blotting is a very important and well-established analytical technique used in cell and molecular biology. It is also known as Protein Immunoblotting due to the use of antibodies to detect its antigen.
This technique is used to detect, analyze and quantify specific proteins on the basis of size, charge, and other differences from the given mixture of various proteins.
History
The work was started in the late 1970s on separating proteins. Towbin in 1979, by using polyacrylamide urea gel, electrophoretically separated proteins. In 1981, Burnette made some improvements and used sodium dodecyl sulfate-polyacrylamide gel.
Steps involved in Western blotting
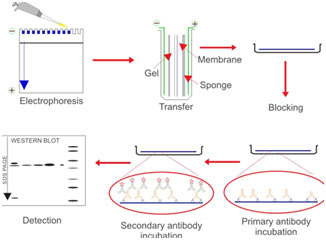
Sample Preparation
First, we separate the protein or extract the protein. Protein can be measured from entire tissue or tissue culture extracts. Cells and tissues ought to be rapidly frozen with liquid nitrogen to prevent protease deterioration of proteins or collected and lysed as quickly as possible.
The quality of proteins is adversely affected by using repeatedly freezing and cycled proteins so it should be avoided.
Lysis Buffers
Numerous cleaning agents, salts, and buffers might be utilized to make it possible for the lysis of cells and to make proteins more soluble. There are various lysis buffers, which can be picked based on the protein of interest.
To prevent the destruction of protein (proteases along with phosphatases can be released during lysis), protease and phosphatase inhibitors can be part of the lysis buffer.
Electrophoretic separation of proteins
Proteins are commonly separated utilizing polyacrylamide gel electrophoresis (PAGE) to identify specific proteins in an intricate sample or to analyze numerous proteins within a single sample.
When integrated with western blotting, PAGE is a powerful analytical tool offering information on the mass, charge, purity, or existence of a protein. Sodium dodecyl sulfate-PAGE, or SDS-PAGE, separates proteins according to mass due to the negative charge imparted on proteins bound to the ionic SDS cleaning agent or detergent.
Transferring proteins to a membrane
Following electrophoresis, the protein needs to be moved from the gel to a membrane. The transfer approach that is most frequently used for proteins is electroelution or electrophoretic transfer because of its speed and transfer efficiency.
This approach uses the electrophoretic movement of proteins to move them from the gel to the membrane. Electrophoretic transfer of proteins includes putting a protein-containing polyacrylamide gel in direct contact with a piece of nitrocellulose or other ideal, protein-binding assistance, and “sandwiching” this in between two electrodes submerged in a conducting solution.
The procedure involves using permeable pads and filter paper to help with the transfer. When an electric field is used, the proteins vacate the polyacrylamide gel and onto the surface area of the membrane, where the proteins become tightly attached. The outcome is a membrane with a copy of the protein pattern that was originally in the polyacrylamide gel.
Primary antibodies
Western blotting is generally performed by penetrating the blocked membrane with a primary antibody that recognizes a particular protein or epitope on a group of proteins (e.g., SH2 domain or phosphorylated tyrosine). The choice of a primary antibody for a western blot will depend on the antigen to be identified and what antibodies are available to that antigen.
Detection
For detection methods, colorimetric, radioactive, and fluorescent methods can be utilized. In Western blotting immunodetection, proteins are identified through their binding to specific antibodies.
Typically, a primary antibody is utilized in combination with an HRP- or AP-conjugated secondary antibody for chemiluminescent or colorimetric detection using a suitable substrate. Additionally, a fluorescently identified primary or secondary antibody can be utilized for direct visualization.
Uses
Western blotting is utilized extensively in biochemistry to identify the existence of specific proteins, to determine the level of post-translational modifications, to validate protein expression in cloning applications, to examine protein and biomarker expression levels, in antibody epitope mapping, and to test for markers of diseases in scientific settings.
It is utilized in the confirmatory tests of HIV and Hepatitis B.
As the definitive test for Bovine spongiform encephalopathy also called mad cow disease.
Also utilized in testing some Lyme diseases.
