Abstract
VSEPR theory typically used to anticipate the geometry of molecules states that lone pairs and bond pairs are arranged around a central atom at a maximum range apart so the repulsions can be minimal.
- The theory says that both lone pairs and bond pairs are involved in determining the geometry of molecule.
- The lone pairs occupy more area than bond pairs.
- The double bond and triple bond have a greater charge density but in identifying the geometry of molecule they act as a single bond pair.
- VSEPR theory discusses the shapes of non-transition element molecules.
In this article, you will learn about different geometries like AB2, AB3, AB4 type molecules identified by VSEPR theory.
- 1) What is the VSEPR Theory?
- 2) Basic Assumption
- 3) Postulates of VSEPR Theory
- 4) Shapes of Molecules According to VSEPR Theory
- 5) Multiple-Choice Questions (MCQs) with Answers:
- 6) Frequently Asked Questions (FAQs) – Valence Shell Electron Pair Repulsion Theory
- 7) Summary: Valence Shell Electron Pair Repulsion Theory Tutorial
- 8) You may also like to learn:
What is the VSEPR Theory?
The Valence Shell Electron Pair Repulsion Theory is often shortened as VSEPR (pronounced “vesper”). It is generally a theory to anticipate the geometry of molecules. Particularly, the VSEPR theory looks at the bonding and molecular geometry of organic molecules and polyatomic ions. It is useful for nearly all substances that have a central atom that is not a metal.
Sidgwick and Powell (1940) explained that the shapes of molecules could be analyzed in terms of electron pairs in the external orbit of the central atom. Nyholm and Gillespie established the VSEPR theory, which discusses the shapes of molecules for non- transition elements.
Basic Assumption
The valence electron pairs (lone pairs and the bond pairs) are set up around the central atom to remain at a maximum range apart to keep repulsions at a minimum.
Postulates of VSEPR Theory
- (i) Both the lone pairs along with the bond pairs participate in figuring out the geometry of the particles.
- (ii) The electron pairs are arranged around the central polyvalent atom so as to remain at a maximum range apart to avoid repulsions.
- (iii) The electron pairs of lone pairs occupy more area than the bond pairs.
A bonding electron pair is brought in by both nuclei of atoms while non-bonding by only one nucleus. Because a lone pair experiences less nuclear attraction, its electronic charge is expanded more in space than that for a bonding pair. As a result, the non-bonding electron pair exerts higher repulsive forces on bonding electron pairs and therefore tends to compress the bond pairs.
The magnitude of repulsions between the electron sets in an offered molecule decreases in the following order:
Lone pair- lone pair > lone pair-bond pair > bond pair-bond pair
These repulsions are called van der Waals repulsions.
- (iv) The two-electron pairs of a double bond and 3 electron pairs of a triple bond, contain a greater electronic charge density. For that reason, they inhabit more space than one electron pair of a single bond but act like a single electron pair in identifying the geometry of the molecule. This is because they tend to occupy the same region between the two nuclei like a single bond.
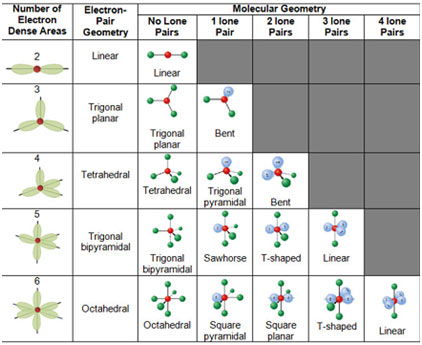
Shapes of Molecules According to VSEPR Theory
1. Molecules Containing Two-Electron Pairs (AB2 type)
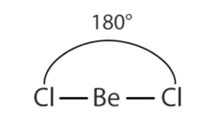
In such, molecules two electrons, pairs around the central atom are set up at a farther range apart at an angle of 180 °, in order to lessen repulsions between them. Therefore, they form a linear geometry.
Beryllium chloride is a common linear molecule, which includes two electrons pairs. MgCl2, CaCl2, SrCl2, CdCL2 and HgCl2 are also linear molecules. The central atoms have 2 electrons in the outermost orbitals.
2. Molecules Containing Three Electron Pairs — (AB3 type)
(a) AB3 Type with no Lone Pairs
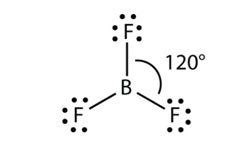
In such molecules, the central atom includes three bonding electron pairs, which are organized at a maximum distance apart at a shared angle of 120 °, providing a triangular planar geometry. The boron atom in BH3 is surrounded by three charge clouds, which remain farthest apart in one plane, each pointing towards the corners of an equilateral triangle.
Therefore, BH3, molecules have a trigonal planar geometry, with each H- B-H bond angles of 120 °. We expect similar geometries in hydrides of group III-A (AlH3, GaH3, InH3, and TlH3) and their halides (BF3, AlCl3, and so on).
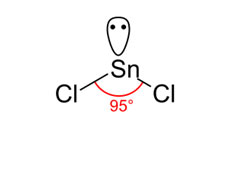
(b) AB3-Type with One Lone Pair and Two Bond Pairs
In SnCl2, one of the corners of the triangle is inhabited by a lone pair, giving rise to a distorted triangular structure in vapor phase.
(c) AB3-Type with MultipleBonds
In SO2, one corner of the triangle is occupied by a lone pair and two corners each by S =O double bond, while in SO3 all 3 corners, each is occupied by S = O bonds. This structure of SO3 is completely triangular.
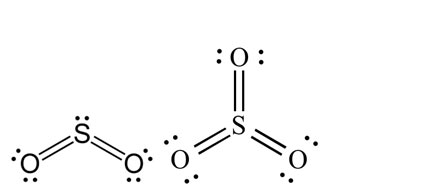
3. Molecules Containing Four Electron Pairs (AB4– Type)
(a) AB4 Type with no Lone Pairs
The charge clouds due to 4 electron pairs avoid their electrostatic repulsions by drifting apart, so to preserve a mutual bond angle of 109.5 °. Such geometry makes it possible for a kinda shape of the regular tetrahedron.
Examples:
Each of the four valence electrons of carbon pair up with the sole electron of hydrogen in methane.
6C = 1s2, 2s1, 2px1, 2py1, 2py1
The 4 electron pairs are directed from the center towards the corners of a regular tetrahedron, with each apex representing a hydrogen nucleus. The arrangement permits a non-planar plane of electron pairs. Each H-C-H bond is perfectly 109.5 °. On the very same premises, SiH4, GeH4, CCl4 type similar geometries. This structure has 4 corners, four faces, six edges, and six bond angles.
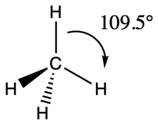
(b) AB4 – Type with One Lone Pair and Three Bond Pairs
In such cases, the charge cloud of lone pair electrons (non-bonding electrons) expands more than that of bonding electrons. As a result, a somewhat large lone pair charge cloud tends to compress the bond angles in the remaining of the molecules.
Ammonia, NH3 is a common example in this case.
![]()
The non-bonding electron in 2s orbital uses up more space and exerts a strong repulsive force on the bonding electron pairs. Consequently, to avoid a larger repulsion, the bonding electron pairs move closer that minimizes the ideal bond angle from 109.50 to 107.5 °. This effect forces ammonia to assume a triangular pyramidal geometry instead of a tetrahedral, as in methane. Similar, effects are evident in the geometries of molecules like PH3, AsH3, SbH3, and BiH3.
The substitution of hydrogen with electronegative atoms like F or Cl further decreases the bond angle.
In NF3, the strong polarity of the N-F bond pulls the lone pair of N atom closer to its nucleus, which in turn puts in a stronger repulsion over bonding electrons. Hence, the angle further diminishes to 102 °. Furthermore, the bond pairs N-F bonds are nearer to F atoms than N atoms. The increased distances in these bond sets make their repulsions less operative.
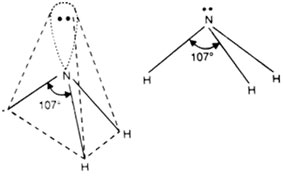
(c) AB4-Type with Two lone Pairs and Two Bond Pairs:
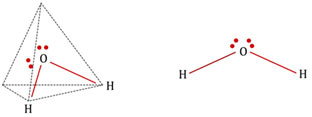
The presence of two lone pairs introduces three types of repulsion i.e. lone pair-lone pair, lone pair-bond pair, and bond pair-bond pair repulsion. For instance: water (H2O), a triatomic molecule is anticipated to be an AB2 type linear molecule like BeCl2 and CO2.
But speculative proofs confirm a bent or angular geometry. VSEPR theory, effectively justifies the speculative results by arguing the participation of lone pairs, in addition to bond pairs in figuring out the general geometry of water molecules.
![]()
2 of the corners of a tetrahedron are inhabited by each of the two lone pairs and staying by bond pairs. But owing to the spatial arrangement of lone pairs and their repulsive action among themselves and on bond pairs, the bond angle is further decreased to 104.5 °. H2S, H2Se, H2Te have similar geometries.
Multiple-Choice Questions (MCQs) with Answers:
- What does VSEPR stand for?
- a) Valence Shell Electron Pair Repulsion Theory
- b) Valence Bond Theory
- c) Van der Waals Theory
- d) Vibration Spectroscopy Electron Pair Repulsion
Answer: a) Valence Shell Electron Pair Repulsion Theory
- What is the primary focus of the VSEPR theory?
- a) Bond Strength
- b) Molecular Weight
- c) Geometry of Molecules
- d) Electron Orbitals
Answer: c) Geometry of Molecules
- Who proposed the Valence Shell Electron Pair Repulsion Theory?
- a) Sidgwick and Powell
- b) Nyholm and Gillespie
- c) Linus Pauling
- d) G.N. Lewis
Answer: b) Nyholm and Gillespie
- According to VSEPR theory, why are lone pairs and bond pairs arranged at a maximum distance apart?
- a) To maximize repulsions
- b) To minimize repulsions
- c) To create attractive forces
- d) To achieve resonance
Answer: b) To minimize repulsions
- Which electron pairs occupy more space according to VSEPR theory?
- a) Bond pairs
- b) Triple bond pairs
- c) Double bond pairs
- d) Lone pairs
Answer: d) Lone pairs
- What does the VSEPR theory state about the direction of electron pairs around the central atom?
- a) They are random
- b) They are fixed
- c) They are at a minimum distance
- d) They are at a maximum distance
Answer: d) They are at a maximum distance
- Which of the following is an example of a linear molecule (AB2 type) according to VSEPR theory?
- a) BH3
- b) BeCl2
- c) NH3
- d) CH4
Answer: b) BeCl2
- In the VSEPR theory, what is the ideal bond angle for molecules containing three electron pairs (AB3 type)?
- a) 90°
- b) 120°
- c) 180°
- d) 109.5°
Answer: b) 120°
- Which molecule exhibits a trigonal pyramidal geometry in the VSEPR theory?
- a) BF3
- b) CO2
- c) NH3
- d) CH4
Answer: c) NH3
- What is the ideal bond angle for molecules containing four electron pairs with no lone pairs (AB4 type)?
- a) 90°
- b) 109.5°
- c) 120°
- d) 180°
Answer: b) 109.5°
- In molecules with two lone pairs and two bond pairs (AB4 type), what is the expected bond angle?
- a) 90°
- b) 104.5°
- c) 120°
- d) 180°
Answer: b) 104.5°
- Which of the following is NOT a postulate of the VSEPR theory?
- a) Lone pair-lone pair repulsion > Lone pair-bond pair repulsion
- b) Bond pair-bond pair repulsion > Lone pair-lone pair repulsion
- c) Bond pair-bond pair repulsion > Lone pair-bond pair repulsion
- d) Lone pair-bond pair repulsion > Bond pair-bond pair repulsion
Answer: d) Lone pair-bond pair repulsion > Bond pair-bond pair repulsion
- What is the role of van der Waals repulsions in VSEPR theory?
- a) Attracting electrons
- b) Minimizing electron density
- c) Reducing repulsions between electron pairs
- d) Creating covalent bonds
Answer: c) Reducing repulsions between electron pairs
- What is the significance of lone pairs in molecules according to VSEPR theory?
- a) They increase bond strength
- b) They decrease bond angles
- c) They affect molecular geometry
- d) They are irrelevant
Answer: c) They affect molecular geometry
- Which theory is useful for substances with a central atom that is not a metal?
- a) Quantum Mechanics
- b) VSEPR Theory
- c) Valence Bond Theory
- d) Atomic Structure Theory
Answer: b) VSEPR Theory
- Who explained the shapes of molecules in terms of electron pairs in the external orbit of the central atom?
- a) G.N. Lewis
- b) Linus Pauling
- c) Sidgwick and Powell
- d) Nyholm and Gillespie
Frequently Asked Questions (FAQs) – Valence Shell Electron Pair Repulsion Theory
- What is the Valence Shell Electron Pair Repulsion (VSEPR) Theory?
- Answer: VSEPR Theory is a model used to predict the geometric shapes of molecules. It posits that electron pairs, both lone and bonded, around a central atom arrange themselves to minimize repulsions.
- Who developed the VSEPR Theory, and when?
- Answer: The theory was established by Nyholm and Gillespie, building on the work of Sidgwick and Powell. It gained prominence in the 20th century.
- What is the basic assumption of the VSEPR Theory?
- Answer: The valence electron pairs (lone pairs and bond pairs) are arranged to stay at a maximum distance apart, minimizing repulsions between them.
- Why do lone pairs occupy more space than bond pairs in the VSEPR Theory?
- Answer: Lone pairs experience less nuclear attraction and, therefore, their electronic charge is expanded more in space compared to bonding pairs, leading to greater repulsive forces.
- What is the significance of van der Waals repulsions in VSEPR Theory?
- Answer: Van der Waals repulsions refer to repulsive forces between electron pairs. These repulsions follow the order: Lone pair-lone pair > Lone pair-bond pair > Bond pair-bond pair.
- How does VSEPR explain the geometry of molecules with multiple bonds, such as double and triple bonds?
- Answer: The electron pairs in double and triple bonds, despite having a greater electronic charge density, act like a single electron pair in determining the geometry of the molecule.
- Which molecules fall under the category of AB2 type in VSEPR Theory, and what is their geometry?
- Answer: Examples include linear molecules like BeCl2 and MgCl2, where two electron pairs are arranged at a maximum distance apart, forming a linear geometry with a bond angle of 180°.
- What is the ideal bond angle for molecules containing three electron pairs with no lone pairs (AB3 type)?
- Answer: The ideal bond angle is 120°, creating a triangular planar geometry. An example is BH3.
- How does VSEPR Theory explain the geometry when a molecule has one lone pair and two bond pairs (AB3 type)?
- Answer: The presence of a lone pair causes a distorted triangular structure, as seen in molecules like SnCl2.
- In molecules with four electron pairs and no lone pairs (AB4 type), what is the ideal bond angle?
- Answer: The ideal bond angle is 109.5°, forming a tetrahedral geometry. Examples include CH4 and SiH4.
- Explain the impact of lone pairs on bond angles in molecules with one lone pair and three bond pairs (AB4 type).
- Answer: Lone pairs exert a stronger repulsive force, causing a compression of bond angles. Ammonia (NH3) is an example with a bond angle of 107.5°.
- What happens to the bond angle in molecules with two lone pairs and two bond pairs (AB4 type)?
- Answer: The bond angle is further decreased to 104.5° due to the repulsion between lone pairs. Examples include H2O.
Summary: Valence Shell Electron Pair Repulsion Theory Tutorial
The Valence Shell Electron Pair Repulsion (VSEPR) Theory is a powerful model used to predict the geometric shapes of molecules based on the arrangement of electron pairs. This tutorial provides a comprehensive understanding of the theory and its applications.
Key Points:
- Abstract:
- VSEPR theory anticipates molecular geometry by arranging lone pairs and bond pairs around a central atom to minimize repulsions.
- Both lone pairs and bond pairs contribute to determining the overall geometry of a molecule.
- What is the VSEPR Theory?
- VSEPR, or Valence Shell Electron Pair Repulsion Theory, is crucial for predicting the geometry of molecules.
- It is applicable to organic molecules and polyatomic ions with a non-metal central atom.
- Developed by Nyholm and Gillespie, it builds on the analysis of electron pairs in the external orbit by Sidgwick and Powell.
- Basic Assumption:
- Valence electron pairs are arranged around the central atom to stay at a maximum range apart, minimizing repulsions.
- Postulates of VSEPR Theory:
- Both lone pairs and bond pairs participate in determining molecular geometry.
- Electron pairs arrange around the central polyvalent atom to remain at a maximum range apart.
- Lone pairs occupy more space than bond pairs, influencing geometry.
- Magnitude of repulsions: Lone pair-lone pair > Lone pair-bond pair > Bond pair-bond pair.
- Multiple bonds (double and triple) act like a single bond in determining geometry.
- Shapes of Molecules According to VSEPR Theory:
- AB2 Type (Two-Electron Pairs):
- Linear geometry (e.g., Beryllium chloride).
- AB3 Type (Three Electron Pairs):
- Triangular planar geometry (e.g., BH3) with variations for lone pairs.
- AB4 Type (Four Electron Pairs):
- Tetrahedral geometry (e.g., CH4) with variations for lone pairs.
- AB4 Type with Lone Pairs:
- Lone pairs compress bond angles (e.g., Ammonia, NH3).
- AB4 Type with Two Lone Pairs:
- Introduces three types of repulsion (e.g., Water, H2O).
- AB4 Type with Three Bond Pairs and One Lone Pair:
- Distorted tetrahedral geometry (e.g., NF3).
- AB2 Type (Two-Electron Pairs):
- Conclusion:
- VSEPR theory is a versatile tool for understanding and predicting molecular geometries, essential for students and researchers in chemistry.
This tutorial offers a comprehensive exploration of VSEPR theory, enhancing the understanding of molecular shapes and structures.
