Thermodynamics and Laws of Thermodynamics
Thermodynamics deals with the principles of heat and temperature and the inter-conversion of heat and other forms of energy. The four laws of thermodynamics govern the behavior of these amounts and offer a quantitative description. William Thomson, in 1749, created the term thermodynamics.
The word “Thermodynamics” is derived from two Greek words “thermes” and “dynamikos” which suggest heat and powerful respectively.
- 1) System or Surroundings
- 2) Types of Systems
- 3) Laws of Thermodynamics
- 4) Zeroth law of thermodynamics
- 5) The First Law of Thermodynamics
- 6) The Second Law of Thermodynamics
- 7) Third Law of Thermodynamics
- 8) MCQs:
- 9) Summary: Thermodynamics and Laws of Thermodynamics
- 10) You may also like to learn:
System or Surroundings
To avoid confusion, researchers discuss thermodynamic values in reference to a system and its surroundings. Everything that is not a part of the system constitutes its surroundings. The system and surroundings are separated by a boundary.
For example, if the system is one mole of a gas in a container, then the boundary is simply the inner wall of the container itself. Whatever beyond the boundary is thought about the surroundings, which would consist of the container itself.
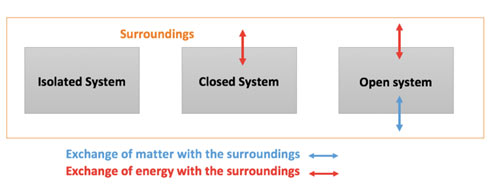
Types of Systems
There are three types of system:
Isolated System
An isolated system cannot exchange both energy and mass with its surroundings. The universe is thought about an isolated system.
Closed System
Throughout the limit of the closed system, the transfer of energy happens however the transfer of mass doesn’t take place. Fridge, and compression of gas in the piston-cylinder assembly are examples of closed systems.
Open System
In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
Laws of Thermodynamics
The laws of thermodynamics define the essential physical amounts like energy, temperature level, and entropy that identify thermodynamic systems at thermal equilibrium. These thermodynamic laws represent how these quantities behave under various situations.
There are four laws of thermodynamics and are provided below:
- Zeroth law of thermodynamics
- The first law of thermodynamics
- The second law of thermodynamics
- Third law of thermodynamics
Zeroth law of thermodynamics
If two thermodynamic systems are each in thermal equilibrium with a third, then they remain in thermal equilibrium with each other.
The First Law of Thermodynamics
The first law of thermodynamics also referred to as the Law of Conservation of Energy, specifies that energy can neither be created nor be destroyed; energy can just be transferred or altered from one kind to another. For example, turning on a light would seem to produce energy; however, it is electrical energy that is transformed.
A method of expressing the first law of thermodynamics is that any change in the internal energy (∆ E) of a system is given by the sum of the heat (q) that streams across its boundaries and the work (w) done on the system by the surroundings:
∆ E = q + w
The Second Law of Thermodynamics
The second law of thermodynamics states that the entropy of any isolated system constantly increases. Isolated systems spontaneously develop towards thermal equilibrium– the state of optimum entropy of the system. More simply put: the entropy of the universe (the ultimate isolated system) just increases and never decreases.
An easy way to think of the second law of thermodynamics is that a room, if not spick-and-span, will usually end up being untidier and more disorderly with time– regardless of how cautious one is to keep it tidy. When space is cleaned, its entropy reduces, however, the effort to clean it has led to a boost in entropy outside the room that surpasses the entropy lost.
Third Law of Thermodynamics
The Third Law of Thermodynamics says that a perfect crystalline structure at absolute zero temperatures will have zero disorder or entropy. Nevertheless, if there is even the tiniest tip of the flaw in this crystalline structure, then there will likewise be a minimal quantity of entropy.
This law gets a little weird though, because even at absolutely zero Kelvin there is still some atomic motion happening, so it’s a bit theoretical. Regardless, this law permits us to comprehend that as the entropy of a system approaches a temperature of absolute zero, the entropy present within a system decreases.
MCQs:
- What is thermodynamics primarily concerned with?
- A) Principles of electricity
- B) Principles of heat and temperature
- C) Principles of magnetism
- D) Principles of mechanics
- Answer: B) Principles of heat and temperature
- Who coined the term “thermodynamics”?
- A) William Thomson
- B) Isaac Newton
- C) Albert Einstein
- D) Galileo Galilei
- Answer: A) William Thomson
- What do thermodynamic researchers refer to when discussing thermodynamic values?
- A) System only
- B) Surroundings only
- C) System and surroundings together
- D) None of the above
- Answer: C) System and surroundings together
- Which of the following is considered an isolated system?
- A) A steam turbine
- B) A closed system
- C) A refrigerator
- D) The universe
- Answer: D) The universe
- According to the zeroth law of thermodynamics, what happens if two thermodynamic systems are each in thermal equilibrium with a third?
- A) They do not remain in thermal equilibrium with each other
- B) They remain in thermal equilibrium with each other
- C) Their temperatures are not equal
- D) They lose thermal equilibrium with each other
- Answer: B) They remain in thermal equilibrium with each other
- What does the first law of thermodynamics state?
- A) Energy can be created but not destroyed
- B) Energy can be destroyed but not created
- C) Energy can neither be created nor destroyed
- D) Energy can be converted into mass
- Answer: C) Energy can neither be created nor destroyed
- According to the second law of thermodynamics, what happens to the entropy of any isolated system?
- A) It decreases
- B) It remains constant
- C) It fluctuates randomly
- D) It constantly increases
- Answer: D) It constantly increases
- What is the third law of thermodynamics concerned with?
- A) Behavior of gases at low temperatures
- B) Behavior of liquids at high temperatures
- C) Behavior of solids at absolute zero
- D) Behavior of plasmas at extreme pressures
- Answer: C) Behavior of solids at absolute zero
- Which Greek words are thermodynamics derived from?
- A) “Heat” and “movement”
- B) “Temperature” and “energy”
- C) “Heat” and “powerful”
- D) “Energy” and “flow”
- Answer: C) “Heat” and “powerful”
- In thermodynamics, what does the system refer to?
- A) Everything outside the boundary
- B) Everything inside the boundary
- C) The boundary itself
- D) The surroundings
- Answer: B) Everything inside the boundary
- Which of the following is an example of an open system?
- A) A gas-filled balloon
- B) A sealed thermos flask
- C) A closed room
- D) A refrigerator
- Answer: A) A gas-filled balloon
- What quantity does the first law of thermodynamics state is conserved?
- A) Heat
- B) Work
- C) Energy
- D) Mass
- Answer: C) Energy
- What is the equation for the first law of thermodynamics?
- A) ∆E = q
- B) ∆E = q + w
- C) ∆E = q/w
- D) ∆E = w/q
- Answer: B) ∆E = q + w
- According to the second law of thermodynamics, what happens to the entropy of the universe?
- A) It decreases
- B) It remains constant
- C) It fluctuates randomly
- D) It increases
- Answer: D) It increases
- What law of thermodynamics is also known as the Law of Conservation of Energy?
- A) The zeroth law
- B) The first law
- C) The second law
- D) The third law
- Answer: B) The first law
- Which law of thermodynamics deals with the concept of thermal equilibrium?
- A) The zeroth law
- B) The first law
- C) The second law
- D) The third law
- Answer: A) The zeroth law
- What does the third law of thermodynamics state about entropy at absolute zero temperature?
- A) It is maximum
- B) It is minimum
- C) It is zero
- D) It is undefined
- Answer: C) It is zero
- Which law of thermodynamics deals with the concept of entropy?
- A) The zeroth law
- B) The first law
- C) The second law
- D) The third law
- Answer: C) The second law
- What is the SI unit of power?
- A) Joule
- B) Watt
- C) Newton
- D) Pascal
- Answer: B) Watt
- In which system does the transfer of energy occur but not the transfer of mass?
- A) Isolated system
- B) Closed system
- C) Open system
- D) None of the above
- Answer: B) Closed system
- Which type of system cannot exchange energy or mass with its surroundings?
- A) Isolated system
- B) Closed system
- C) Open system
- D) None of the above
- Answer: A) Isolated system
Summary: Thermodynamics and Laws of Thermodynamics
Thermodynamics is the branch of physics dealing with heat, temperature, and energy, including their inter-conversion and transfer. The term “thermodynamics” was coined by William Thomson in 1749, deriving from the Greek words “thermes” (heat) and “dynamikos” (powerful).
System or Surroundings:
- Thermodynamic principles are often discussed in terms of systems and surroundings, with the system referring to the portion under study and the surroundings encompassing everything else.
- Systems can be classified into isolated, closed, or open systems based on their ability to exchange energy and mass with the surroundings.
Laws of Thermodynamics:
- Zeroth Law of Thermodynamics: If two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
- First Law of Thermodynamics: Also known as the Law of Conservation of Energy, it states that energy cannot be created or destroyed; it can only change forms or be transferred. Mathematically expressed as ∆E = q + w.
- Second Law of Thermodynamics: Entropy in an isolated system tends to increase over time, moving towards thermal equilibrium. This law describes the irreversible nature of natural processes.
- Third Law of Thermodynamics: At absolute zero temperature, a perfect crystalline structure has zero entropy. It provides insights into the behavior of entropy as systems approach absolute zero.
Understanding these laws provides a foundation for analyzing and predicting the behavior of thermodynamic systems and processes, essential in various fields such as engineering, chemistry, and environmental science.

