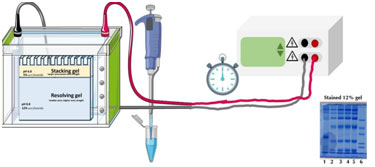
Introduction
SDS – PAGE, Sodium dodecyl sulfate-polyacrylamide gel electrophoresis is an analytical technique widely used in forensics, molecular biology, biochemistry, and genetics to separate protein molecules on basis of their molecular weight and electrophoretic mobility.
What is SDS?
SDS – Sodium dodecyl sulfate which is also known as lauryl sulfate is an anionic detergent. It has a strong protein-denaturing effect. SDS denatures secondary and non-disulfide linked tertiary structures by binding to the protein backbone at a constant molar ratio.
In doing this, SDS confers net negative charge to the polypeptide in portion to its length. Complex structures of proteins are destroyed by negative charges on SDS. Due to their negative charge, they are attracted toward anode in an electric field.
When a detergent SDS is combined with PAGE, it becomes SDS-PAGE. The binding of negative charges on SDS masks the charge of the protein. Hence migrate along with the gel in order of increasing sizes or molecular weights.
Principle of SDS – PAGE
When proteins are separated by electrophoresis through a gel matrix, smaller proteins migrate quicker due to less resistance from the gel matrix. Other influences on the rate of migration through the gel matrix consist of the structure and charge of the proteins.
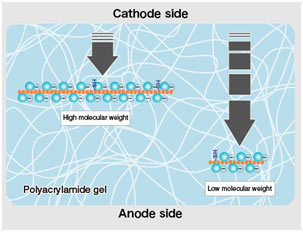
In SDS-PAGE, making use of sodium dodecyl sulfate and polyacrylamide gel mainly removes the influence of the structure and charge, and proteins are separated entirely based upon polypeptide chain length.
Polymerized acrylamide (polyacrylamide) forms a mesh-like matrix appropriate for the separation of proteins of common size. The strength of the gel enables simple handling.
Working of SDS – PAGE
In this polyacrylamide gel is used. Denatured proteins can be filled into this gel, run through it when subjected to an electrical current, however will move at a speed determined by the percentage of acrylamide used to make the gel and will remain in the gel.
Once the current is stopped and the gel is fixed by means of chemical treatments. The larger the protein, the lower the portion of acrylamide is mixed into the gel to deal with distinctions in protein size.
After this, the proteins are fixed within the gel using a series of chemical solutions containing ethanol, acetic acid, and glutaraldehyde to prevent their additional migration out of the gel.
Visualization of Results
After electrophoresis, protein separation cannot be directly observed by the naked eye, and subsequent staining methods are required. Coomassie’s brilliant blue staining and silver staining prevail approaches for routine detection and quantification of proteins separated by electrophoresis.
With the improvement of high-sensitivity protein analysis methods and protein recognition technologies, new staining methods such as fluorescent labeling and isotope labeling innovation have actually significantly improved the level of sensitivity.
Uses of SDS – PAGE
This technique is widely used for the determination of molecular mass, abundance of proteins in a sample, to detect a specific type of protein in the sample containing a mixture of proteins.
By using this, proteins can be purified.
Techniques such as Western blotting, peptide mapping, two-dimensional electrophoresis use this to detect, separate, and analyze proteins.
This is an easy, relatively inexpensive, and more precise method for protein analysis.