An Introduction to Quantum Numbers
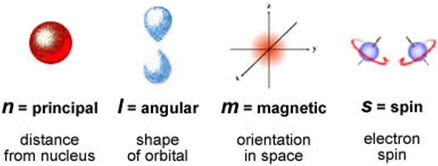
The set of numbers used to explain the position as well as the energy of the electron in an atom are called quantum numbers. These explain the movement and trajectories of each electron within an atom. Quantum numbers are the sets of mathematical values that give the appropriate solutions to the Schrodinger wave formula for the hydrogen atom.
An electron in an atom is entirely described by its four quantum numbers. You understand that a complete address of a person comprises his name, the city in which he lives, the block, the road, and also that’s personal home number. On similar grounds, quantum numbers work as identification numbers or labels, which describe an electron. These quantum numbers specify the position of an electron in an atom.
The Four Quantum Numbers
There are 4 quantum numbers that can describe the electron completely.
- Principal quantum number (n)
- Azimuthal quantum number (ℓ)
- Magnetic quantum number (m)
- Spin quantum number (s)
All quantum numbers are defined and listed below in this post.
Principal Quantum Number (n)
The different energy levels in Bohr’s atom are represented by ‘n’. This is called the primary quantum number by Schrodinger. Its values are non-zero, positive integers up to infinity.
n = 1, 2, 3, 4, 5, … … … …
The value of n represents the shell or energy level in which the electron moves around the nucleus. Letter symbols K, L, M, N, and so on are likewise used to represent the different shells. For example, when n =1, it is called K shell, for n = 2, it is L shell, and so forth.
The values of n additionally establish the location of an electron in an atom, i.e., the distance of the electron from the nucleus, the greater the value of ‘n’ better will certainly be the range of electron from the core. It is a quantitative measure of the dimension of an electronic shell, ‘n’ likewise offers us the energy of the electron in a shell. Bohr’s results aid us in recognizing the relationships between the distance and energy of electrons.
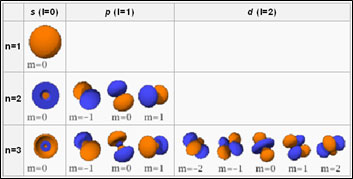
Azimuthal Quantum Number (ℓ)
It has currently been stated in the issues of Bohr’s version that a spectrometer of high resolving power shows that an individual line in the range is more split into several extremely fine lines. This point can be discussed by saying that each shell is separated right into subshells. So, just the primary quantum number (n) is not sufficient to discuss the line range. There is one more subsidiary quantum number called the azimuthal quantum number which is used to represent the subshells. The values of azimuthal quantum number (ℓ).
(ℓ) = 0, 1, 2, 3, … … … … … … … … … … … (n-1)
These values represent different subshells, which are assigned by small letters, s, p, d, f. They mean sharp, primary, diffused, and fundamental, respectively. These are the spectral terms utilized to describe the series of lines observed in the atomic spectrum. The values of azimuthal quantum numbers always start from zero.
A subshell might have different shapes relying on the value of (ℓ). It may be round, dumbbell, or a few other complex shapes. The value of ‘ℓ’ is related to the form of the subshell as adheres to:
| ℓ | s-subshell | spherical | |
| ℓ | 1 | p-subshell | dumb-bell |
| ℓ | 2 | d-subshell | complicated form |
The Magnetic Quantum Number (m)
The magnetic quantum number ml determines the variety of orbitals and also their alignment within a subshell. Consequently, its value depends on the orbital angular momentum quantum number l. Offered a specific l, ml is an interval varying from– l to +l, so it can be zero, an unfavorable integer, or a positive integer.
m = 0, ± 1, ± 2, ± 3, … … … ……
Spin Quantum Number (s)
Alkali metals have one electron in their outer shell. We can record their emission spectra when the outermost electron leaps from an excited state to a ground state. When the spectra are observed using a high settling power spectrometer, each line in the spectrum is found to contain pair of lines, this is called a doublet line framework.
We need to keep in mind, that the doublet line framework is different from the fine spectrum of hydrogen.
In 1925, Goudsmit and Uhlenbech suggested that an electron while moving in an orbital around the nucleus likewise rotates or spins concerning its own axis either in a clockwise or anti-clockwise direction.
This is additionally called self-rotation. This spinning electron is related to a magnetic field and therefore a magnetic moment. Hence, opposite magnetic fields are produced by the clockwise and anti-clockwise rotates of electrons. This spin activity is in charge of the doublet line structure in the spectrum.
MCQs about Quantum Numbers
- 1. Which set of numbers is used to describe the position and energy of an electron in an atom?
- A) Prime numbers
- B) Quantum numbers
- C) Atomic numbers
- D) Complex numbers
- Answer: B) Quantum numbers
- 2. How many quantum numbers are needed to completely describe an electron in an atom?
- A) Two
- B) Three
- C) Four
- D) Five
- Answer: C) Four
- 3. What does the principal quantum number (n) represent?
- A) The shape of the subshell
- B) The number of orbitals within a subshell
- C) The energy level of the electron
- D) The spin of the electron
- Answer: C) The energy level of the electron
- 4. Which quantum number is used to represent the subshells within a principal energy level?
- A) Principal quantum number (n)
- B) Azimuthal quantum number (ℓ)
- C) Magnetic quantum number (m)
- D) Spin quantum number (s)
- Answer: B) Azimuthal quantum number (ℓ)
- 5. What are the possible values of the azimuthal quantum number (ℓ)?
- A) 0, 1, 2, 3, …
- B) 1, 2, 3, 4, …
- C) 0, ±1, ±2, ±3, …
- D) 1, ±1, ±2, ±3, …
- Answer: C) 0, ±1, ±2, ±3, …
- 6. How are the subshells represented according to the azimuthal quantum number (ℓ)?
- A) s, p, d, f
- B) K, L, M, N
- C) 1s, 2p, 3d, 4f
- D) sharp, principal, diffused, fundamental
- Answer: A) s, p, d, f
- 7. What does the magnetic quantum number (m) determine?
- A) The energy level of the electron
- B) The number of electrons in an orbital
- C) The number of orbitals and their alignment within a subshell
- D) The shape of the subshell
- Answer: C) The number of orbitals and their alignment within a subshell
- 8. What are the possible values of the magnetic quantum number (m)?
- A) 0, 1, 2, 3, …
- B) 0, ±1, ±2, ±3, …
- C) 1, 2, 3, 4, …
- D) 1, ±1, ±2, ±3, …
- Answer: B) 0, ±1, ±2, ±3, …
- 9. What phenomenon is responsible for the doublet line structure observed in spectra?
- A) Orbital angular momentum
- B) Spin of electrons
- C) Orbital rotation
- D) Magnetic moment
- Answer: B) Spin of electrons
- 10. Who proposed the concept of electron spin?
- A) Bohr
- B) Schrödinger
- C) Goudsmit and Uhlenbeck
- D) Heisenberg
- Answer: C) Goudsmit and Uhlenbeck
- 11. Which quantum number specifies the shape of the subshell?
- A) Principal quantum number (n)
- B) Azimuthal quantum number (ℓ)
- C) Magnetic quantum number (m)
- D) Spin quantum number (s)
- Answer: B) Azimuthal quantum number (ℓ)
- 12. What are the possible values of the principal quantum number (n)?
- A) Non-zero, positive integers up to infinity
- B) 0, ±1, ±2, ±3, …
- C) 0, 1, 2, 3, …
- D) 1, ±1, ±2, ±3, …
- Answer: C) 0, 1, 2, 3, …
- 13. How is the energy of an electron in a shell related to the principal quantum number (n)?
- A) Energy decreases with increasing n
- B) Energy increases with increasing n
- C) Energy is constant regardless of n
- D) Energy is unrelated to n
- Answer: B) Energy increases with increasing n
- 14. Which of the following represents the order of subshells based on increasing energy?
- A) s, p, d, f
- B) f, d, p, s
- C) p, s, d, f
- D) s, d, p, f
- Answer: A) s, p, d, f
- 15. What is the maximum number of electrons that can occupy an orbital with quantum numbers n = 3 and ℓ = 2?
- A) 2
- B) 6
- C) 8
- D) 18
- Answer: C) 8
- 16. In which subshell would you find the electron with quantum numbers n = 4 and ℓ = 2?
- A) s
- B) p
- C) d
- D) f
- Answer: C) d
- 17. How many orbitals are there in the p subshell?
- A) 1
- B) 2
- C) 3
- D) 4
- Answer: C) 3
- 18. What is the maximum number of electrons that can occupy the subshell with ℓ = 3?
- A) 2
- B) 6
- C) 10
- D) 14
- Answer: D) 14
- 19. What does the spin quantum number (s) determine?
- A) The energy level of the electron
- B) The shape of the subshell
- C) The direction of electron spin
- D) The number of orbitals within a subshell
- Answer: C) The direction of electron spin
- 20. What are the possible values of the spin quantum number (s)?
- A) 0, 1, 2, 3, …
- B) 0, ±1, ±2, ±3, …
- C) 1, 2, 3, 4, …
- D) 1/2, -1/2
- Answer: D) 1/2, -1/2
- 21. How does the value of the azimuthal quantum number (ℓ) affect the shape of the subshell?
- A) It determines the energy level of the electron
- B) It determines the number of electrons in the subshell
- C) It determines the size of the subshell
- D) It determines the shape of the subshell
- Answer: D) It determines the shape of the subshell
- 22. What term is used to describe the set of values observed in the atomic spectrum based on azimuthal quantum numbers?
- A) Principal
- B) Sharp
- C) Diffused
- D) Fundamental
- Answer: B) Sharp
- 23. What is the maximum number of electrons that can occupy an orbital with quantum numbers n = 2 and ℓ = 1?
- A) 2
- B) 4
- C) 6
- D) 8
- Answer: B) 4
- 24. Which quantum number specifies the number of orbitals and their alignment within a subshell?
- A) Principal quantum number (n)
- B) Azimuthal quantum number (ℓ)
- C) Magnetic quantum number (m)
- D) Spin quantum number (s)
- Answer: C) Magnetic quantum number (m)
- 25. What is the interval of possible values for the magnetic quantum number (m) given a specific value of the azimuthal quantum number (ℓ)?
- A) 0 to n
- B) -ℓ to +ℓ
- C) 0 to ℓ
- D) 0 to infinity
- Answer: B) -ℓ to +ℓ
Summary
In this tutorial, we explored the fundamental concept of quantum numbers and their significance in describing the properties and behavior of electrons within an atom. Quantum numbers are sets of mathematical values that provide solutions to the Schrödinger wave equation for the hydrogen atom, allowing for a comprehensive understanding of electron movement and trajectories.
There are four main quantum numbers: principal quantum number (n), azimuthal quantum number (ℓ), magnetic quantum number (m), and spin quantum number (s). Each of these numbers plays a crucial role in defining the characteristics of electrons within an atom.
The principal quantum number (n) determines the energy levels or shells in which electrons orbit around the nucleus, with higher values of n corresponding to greater distances from the nucleus. The azimuthal quantum number (ℓ) further subdivides the shells into subshells or orbitals, denoted by the letters s, p, d, and f, each representing different shapes.
The magnetic quantum number (m) specifies the number of orbitals within a subshell and their alignment, while the spin quantum number (s) describes the direction of electron spin, contributing to phenomena such as spectral line splitting.
Overall, understanding quantum numbers provides a foundational framework for comprehending the behavior of electrons in atoms, guiding our exploration of atomic structure and properties.