Definition of Polymers
A polymer is a big particle or a macromolecule which essentially is a combination of numerous subunits.
The term polymer in Greek means ‘many parts’. Polymers can be found all around us.
From the hair of our DNA which is a naturally occurring biopolymer to polypropylene which is utilized throughout the world as plastic.
Polymers may be naturally found in plants and animals (natural polymers) or might be manufactured (synthetic polymers).
Various polymers have a variety of distinct physical and chemical properties due to which they have great use in daily life.
- 1) The Structure of Polymers
- 2) Classification of Polymers
- 3) Classification of Polymers based on the Source of Availability
- 4) Polymers based upon the Structure of the Monomer Chain
- 5) Classification Based Upon Polymerization
- 6) Attributes of Polymers
- 7) Some Polymers and their Monomers
- 8) Multiple-Choice Questions (MCQs) with Answers: Polymers
- 9) Frequently Asked Questions (FAQs) – Polymers
- 10) Concluding Polymers Tutorial
- 11) You may also like to learn:
The Structure of Polymers
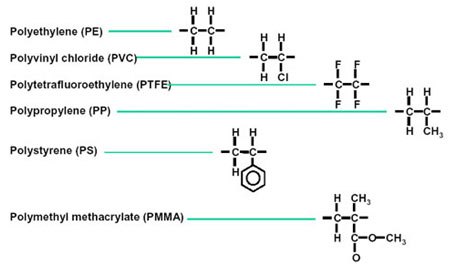
Lots of typical classes of polymers are made up of hydrocarbons, compounds of carbon and hydrogen. These polymers are particularly made of carbon atoms bonded together, one to the next, into long chains that are called the backbone of the polymer. Because of the nature of carbon, several other atoms can be connected to each carbon atom in the chain. There are polymers that contain only carbon and hydrogen atoms.
Polyethylene, polypropylene, polybutylene, polystyrene, and polymethyl pentene are examples of these. Polyvinyl chloride (PVC) has actually chlorine attached to the all-carbon chains. Teflon has actually fluorine attached to the all-carbon backbone.
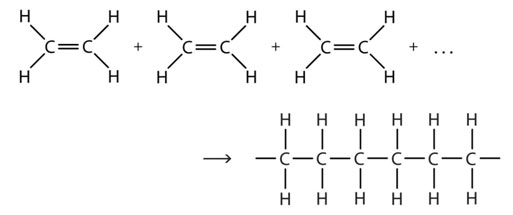
Other typical made polymers have foundations that consist of aspects aside from carbon. Nylons contain nitrogen atoms in the repeat unit backbone. Polyesters and polycarbonates include oxygen in the backbone. There are also some polymers that, instead of having a carbon chain, have silicon or phosphorous backbone. These are considered inorganic polymers.
Classification of Polymers
Polymers cannot be classified under one category because of their complex structures, various behaviors, and huge applications. We can, therefore, classify polymers based upon the following considerations.
Classification of Polymers based on the Source of Availability
There are 3 kinds under this classification, specifically, Natural, Artificial, and Semi-synthetic Polymers.
Natural Polymers:
They occur naturally and are found in plants and animals. For instance, proteins, starch, cellulose, and rubber. To add more, there also have eco-friendly polymers which are called biopolymers.
Semi-synthetic Polymers:
They are originated from naturally occurring polymers and go through further chemical changes. For instance, cellulose nitrate, cellulose acetate.
Synthetic Polymers:
These are man-made polymers. Plastic is the most common and commonly utilized synthetic polymer. It is used in industries and various dairy products. For example, nylon-6, 6, polyether’s, and so on.
Polymers based upon the Structure of the Monomer Chain
This category has the following classifications:
Linear Polymers
The structure of polymers containing long and straight chains falls under this category. PVC, i.e., polyvinyl chloride is mainly utilized for making pipes and electrical cable televisions is an example of a linear polymer.
Branched-chain Polymers
When straight chains of a polymer form branch, then, such polymers are categorized as branched-chain polymers. For example, Low-density polythene.
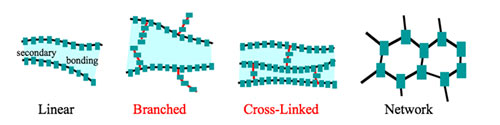
Cross-linked Polymers
They are made up of bifunctional and trifunctional monomers. They have a more powerful covalent bond in comparison to other linear polymers. Bakelite and melamine are examples in this category.
Classification Based Upon Polymerization
- Addition Polymerization: Example, poly ethane, Teflon, Polyvinyl chloride (PVC).
- Condensation Polymerization: Example, Nylon -6, 6, perylene, polyesters.
Category Based Upon Monomers
- Homomer: In this type, a single kind of monomer unit is present. For instance, Polyethene.
- Heteropolymer or co-polymer: It consists of different kind of monomer units. For instance, nylon -6, 6.
Category Based Upon Molecular Forces
- Elastomers: These are rubber-like solids weak interaction forces exist. For example, Rubber.
- Fibers: Strong, hard, high tensile strength and strong forces of interaction exist. For example, nylon -6, 6.
- Thermoplastics: These have intermediate forces of destination. For example, polyvinyl chloride.
- Thermosetting polymers: These polymers greatly improve the material’s mechanical properties. It offers enhanced chemical and heat resistance. For instance, phenolics, epoxies, and silicones.
Attributes of Polymers
Every polymer has really unique characteristics, but the majority of polymers have the following general attributes.
- Polymers can be really resistant to chemicals.
- Polymers can be both thermal and electrical insulators.
- Typically, polymers are extremely light in weight with significant degrees of strength.
- Polymers can be processed in different methods.
- Polymers are products with a relatively limitless variety of attributes and colors.
- Polymers are usually made from petroleum, but not always.
- Polymers can be used to make things that have no alternatives from other products.
Some Polymers and their Monomers
- Polypropene, also called polypropylene, is comprised of monomer propene.
- Polystyrene is an aromatic polymer, naturally transparent, made up of monomer styrene.
- Polyvinyl chloride (PVC) is a plastic polymer made from monomer vinyl chloride.
- The urea-formaldehyde resin is a non-transparent plastic obtained by heating formaldehyde and urea.
- Glyptal is comprised of monomers ethylene glycol and phthalic acid.
- Bakelite or polyoxybenzylmethylenglycolanhydride is a plastic that is made up of monomers phenol and aldehyde.
Multiple-Choice Questions (MCQs) with Answers: Polymers
- What is a polymer?
- A. Small particle
- B. Macromolecule
- C. Atom
- D. Compound
- Answer: B
- What does the term “polymer” mean in Greek?
- A. Single part
- B. Complex structure
- C. Many parts
- D. Long chain
- Answer: C
- Which of the following is a naturally occurring biopolymer?
- A. Polyethylene
- B. Polypropylene
- C. DNA
- D. PVC
- Answer: C
- What type of bonds make up the backbone of hydrocarbon-based polymers?
- A. Ionic bonds
- B. Covalent bonds
- C. Hydrogen bonds
- D. Metallic bonds
- Answer: B
- Which polymer has fluorine attached to the all-carbon backbone?
- A. Polyethylene
- B. Polypropylene
- C. PVC
- D. Teflon
- Answer: D
- In which classification do rubber-like solids with weak interaction forces belong?
- A. Elastomers
- B. Fibers
- C. Thermoplastics
- D. Thermosetting polymers
- Answer: A
- What is an example of a branched-chain polymer?
- A. Polyethylene
- B. Polypropylene
- C. PVC
- D. Low-density polythene
- Answer: D
- Which classification is based on the source of availability?
- A. Classification based on polymerization
- B. Classification based on the structure of the monomer chain
- C. Classification based on molecular forces
- D. Classification based on the source of availability
- Answer: D
- What is an example of a semi-synthetic polymer?
- A. Polyethylene
- B. Cellulose nitrate
- C. Polypropylene
- D. Nylon-6, 6
- Answer: B
- Which attribute is common to most polymers?
- A. Conductivity
- B. Opacity
- C. Insulation
- D. Weightiness
- Answer: C
- Polyvinyl chloride (PVC) is mainly used for making:
- A. Electrical cables
- B. Pipes
- C. Both A and B
- D. None of the above
- Answer: C
- What is the primary classification based on polymerization?
- A. Addition Polymerization
- B. Condensation Polymerization
- C. Homomer
- D. Heteropolymer
- Answer: A
- Which polymerization process involves the elimination of water or another simple molecule?
- A. Addition Polymerization
- B. Condensation Polymerization
- C. Homomer
- D. Heteropolymer
- Answer: B
- What is the category based upon molecular forces that includes rubber-like solids?
- A. Elastomers
- B. Fibers
- C. Thermoplastics
- D. Thermosetting polymers
- Answer: A
- Which polymer is commonly used in making pipes and electrical cables?
- A. PVC
- B. Polypropylene
- C. Polystyrene
- D. Polyvinyl acetate
- Answer: A
- What is the category of polymers that have intermediate forces of attraction?
- A. Elastomers
- B. Fibers
- C. Thermoplastics
- D. Thermosetting polymers
- Answer: C
- Which classification is based on the structure of the monomer chain?
- A. Classification based on polymerization
- B. Classification based on molecular forces
- C. Classification based on the structure of the monomer chain
- D. Classification based on the source of availability
- Answer: C
- What is an example of a natural polymer?
- A. Nylon-6, 6
- B. Polyethylene
- C. Rubber
- D. Polyvinyl chloride (PVC)
- Answer: C
- Polymerization that involves the combination of a single type of monomer is called:
- A. Addition Polymerization
- B. Condensation Polymerization
- C. Homomer
- D. Heteropolymer
- Answer: C
- Which classification involves polymers derived from naturally occurring polymers undergoing further chemical changes?
- A. Natural Polymers
- B. Synthetic Polymers
- C. Semi-synthetic Polymers
- D. Linear Polymers
- Answer: C
- What is an example of a synthetic polymer?
- A. Cellulose
- B. Starch
- C. Polypropylene
- D. Rubber
- Answer: C
- What are polymers made from petroleum called?
- A. Petro-polymers
- B. Oil-polymers
- C. Petrochemical polymers
- D. Petroleum-based polymers
- Answer: D
- Which polymer is comprised of monomers phenol and aldehyde?
- A. Polyethylene
- B. Polystyrene
- C. Bakelite
- D. Polypropylene
- Answer: C
Frequently Asked Questions (FAQs) – Polymers
1. What is a polymer?
- A polymer is a large molecule or macromolecule formed by the combination of numerous subunits. The term “polymer” in Greek means ‘many parts.’
2. Where can polymers be found in our daily lives?
- Polymers are ubiquitous and can be found in various forms, from the DNA in our hair (a naturally occurring biopolymer) to common plastics like polypropylene used worldwide.
3. How are polymers classified based on the source of availability?
- Polymers are classified into Natural Polymers (occurring in plants and animals), Semi-synthetic Polymers (derived from natural polymers with chemical modifications), and Synthetic Polymers (man-made polymers, e.g., plastic).
4. Can you provide examples of natural polymers?
- Yes, examples of natural polymers include proteins, starch, cellulose, and rubber. Biopolymers are eco-friendly polymers found in nature.
5. What are the categories based on the structure of the monomer chain?
- Polymers are categorized as Linear Polymers (long and straight chains), Branched-chain Polymers (chains with branches), and Cross-linked Polymers (with strong covalent bonds).
6. What are Elastomers and an example of this category?
- Elastomers are rubber-like solids with weak interaction forces. An example is rubber.
7. How are polymers classified based on polymerization?
- Polymers are classified into Addition Polymerization (e.g., polyethylene) and Condensation Polymerization (e.g., nylon-6, 6).
8. What are the general attributes of polymers?
- Polymers are generally resistant to chemicals, serve as thermal and electrical insulators, lightweight yet strong, versatile in processing, available in various colors, often petroleum-based, and essential for making unique products.
9. Can you provide examples of synthetic polymers and their monomers?
- Examples include Polypropene (propene monomer), Polystyrene (styrene monomer), and Polyvinyl Chloride (PVC, vinyl chloride monomer).
10. How are polymers categorized based on molecular forces?
- Categories based on molecular forces include Elastomers, Fibers (with high tensile strength), Thermoplastics (intermediate forces), and Thermosetting polymers (enhanced mechanical properties).
11. Are there alternatives to polymers made from petroleum?
- While polymers are often petroleum-based, there is ongoing research into eco-friendly alternatives and biopolymers derived from renewable sources.
12. How do polymers contribute to everyday life?
- Polymers play a crucial role in daily life by providing materials with diverse properties, from packaging materials and textiles to medical devices and electronics.
13. What is the role of Bakelite, and what are its monomers?
- Bakelite is a plastic with monomers phenol and aldehyde. It is known for its use in electrical insulators and various molded products.
14. Can you explain the difference between addition and condensation polymerization?
- Addition polymerization involves the repeated addition of monomers without the elimination of any byproduct. In contrast, condensation polymerization eliminates a simple molecule, such as water, during the polymerization process.
15. Are polymers recyclable?
- Many polymers are recyclable, contributing to sustainable practices. However, the recyclability depends on the specific type of polymer and the recycling infrastructure in place.
16. How do polymers impact the environment?
- The environmental impact of polymers varies. While some polymers contribute to pollution, research is ongoing to develop environmentally friendly alternatives and recycling methods.
17. Can polymers be used in medical applications?
- Yes, polymers play a vital role in medical applications, including drug delivery systems, medical devices, and tissue engineering.
18. Are there any biodegradable polymers?
- Yes, biodegradable polymers, such as polylactic acid (PLA), are designed to break down naturally, reducing environmental impact.
19. What is the significance of the monomer structure in polymer properties?
- The monomer structure influences the physical and chemical properties of polymers, determining characteristics such as strength, flexibility, and thermal stability.
20. How are polymers involved in the food industry?
- Polymers are used in food packaging materials, ensuring food safety, preserving freshness, and providing convenience in storage and transportation.
21. Are there any polymers with conductive properties?
- Conductive polymers, such as polyaniline, are designed for applications in electronics and sensors due to their electrical conductivity.
Concluding Polymers Tutorial
This comprehensive tutorial delves into the world of polymers, covering their definition, structure, classification, and attributes.
- Definition of Polymers:
- A polymer, a macromolecule with many subunits, is ubiquitous, from DNA’s biopolymer to the global use of polypropylene.
- Structure of Polymers:
- Carbon-based chains form backbones, with variations like chlorine, fluorine, nitrogen, or oxygen in different polymers.
- Classification of Polymers:
- Based on Source:
- Natural Polymers (e.g., proteins, cellulose)
- Semi-synthetic Polymers (e.g., cellulose nitrate)
- Synthetic Polymers (e.g., nylon-6, 6)
- Based on Monomer Chain Structure:
- Linear Polymers (e.g., PVC)
- Branched-chain Polymers (e.g., Low-density polythene)
- Cross-linked Polymers (e.g., Bakelite)
- Based on Polymerization:
- Addition Polymerization (e.g., polyethylene)
- Condensation Polymerization (e.g., Nylon -6, 6)
- Based on Molecular Forces:
- Elastomers (e.g., Rubber)
- Fibers (e.g., nylon -6, 6)
- Thermoplastics (e.g., polyvinyl chloride)
- Thermosetting Polymers (e.g., phenolics)
- Based on Source:
- Attributes of Polymers:
- Resistant to chemicals, thermal and electrical insulators, lightweight, strong, versatile processing, diverse colors and properties.
- Some Polymers and their Monomers:
- Polypropene (monomer: propene), Polystyrene (monomer: styrene), PVC (monomer: vinyl chloride), Urea-formaldehyde resin, Glyptal (monomers: ethylene glycol, phthalic acid), Bakelite (monomers: phenol, aldehyde).
This tutorial provides a comprehensive understanding of polymers, from their fundamental definitions to their diverse applications and characteristics.