What is plasma?
Plasma is typically called the “fourth state of matter”, the other three being solid, liquid and gas. Plasma was identified by the English researcher William Crookes in 1879. In addition to being very important in lots of elements of our everyday life, plasmas are estimated to constitute more than 99 percent of the visible universe. Although naturally taking place plasma is rare in the world, there are numerous manufactured examples.
Inventors have used plasma to conduct electrical energy in neon signs and fluorescent bulbs. Researchers have constructed special chambers to experiment with plasma in laboratories. It occurs only in lightning discharges and in artificial gadgets like fluorescent lights, neon indications, etc. It is all over in our space environment.
How is Plasma formed?
When more heat is supplied, the atoms or molecules might be ionized. An electron may acquire enough energy to leave its atom. This atom loses one electron and develops a net positive charge. It becomes an ion. In a sufficiently heated gas, ionization takes place a lot of times, producing clouds of totally free electrons and ions. However, all the atoms are not necessarily ionized, and some of them may remain entirely intact without any net charge. This ionized gas mixture, consisting of ions, electrons, and neutral atoms is called plasma.
It implies that a plasma is a unique state of matter containing a significant number of electrically charged particles a number sufficient to affect its electrical properties and behavior.
Natural and Artificial Plasma
Artificial plasma can be produced by ionization of gas as in neon signs. Plasma at low temperatures is hard to maintain because outside a vacuum low-temperature plasma reacts rapidly with any molecule it experiences. This aspect makes this material, both very helpful and hard to use. Natural plasma exists only at very high temperatures, or low-temperature vacuums. Natural plasma on the other hand do not break down or react quickly, however is exceptionally hot (over 20,000 ° C minimum). Their energy is so high that they vaporize any product they touch.

Characteristics of Plasma:
- A plasma must have a sufficient variety of charged particles so as a whole, it shows a collective action to electric and magnetic fields. The motion of the particles in the plasma generates fields and electric currents within plasma density. It describes the density of the charged particles. This complex set of interactions makes plasma a special, remarkable, and complex state of matter.
- Although plasma consists of electrons and ions and conducts electrical power, it is macroscopically neutral. In measurable quantities the number of electrons and ions are equivalent.
Where is Plasma found?
The whole universe is almost plasma. It existed before any other type of matter came into being. Plasmas are found in everything from the sun to quarks, the tiniest particles in the universe. As specified earlier plasma is the most abundant form of matter in the universe. It is the stuff of stars. A bulk of the matter in inner-stellar space is plasma. All the stars that shine are all plasma. The sun is a 1.5-million-kilometer ball of plasma, warmed by nuclear fusion.

On Earth, it is just found in a couple of limited regions, like lightning bolts, flames, auroras, and fluorescent lights. When an electric current is passed through neon gas, it produces both plasma and light.
Applications of Plasma:
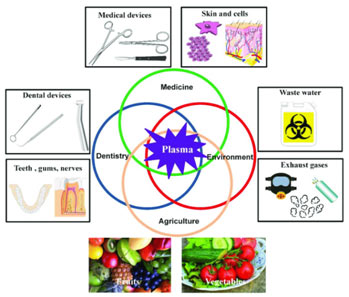
Plasma has numerous crucial technological applications. It is present in numerous devices. It helps us to comprehend much of the universe around us. Since plasmas are conductive react to electric and magnetic fields and can be efficient sources of radiation, so they can be utilized in many applications where such control is needed or when unique sources of energy or radiation are required.
- A fluorescent light bulb is not like a routine light bulb. Inside the long tube is a gas. When the light is switched on, electrical energy flows through the tube. This electrical power serves as that special energy and charges up the gas. This charging and excitement of the atoms develop a radiant plasma inside the bulb.
- Neon signs are glass tubes filled with gas. When they are turned on then the electrical energy flows through the tube. The electrical power charges the gas, potentially neon, and creates a plasma inside the tube. The plasma glows with a unique color depending on what type of gas is inside.
- They find applications such as plasma processing of semiconductors, sanitation of some medical products, lamps, lasers, diamond layered films, high power microwave sources, and pulsed power switches.
- They also provide the foundation for important prospective applications such as the generation of electrical energy from fusion, pollution control, and removal of hazardous chemicals.
- Plasma lights up our offices and houses, makes our computers and electronic devices work.
- They drive lasers and particle accelerators, help to tidy up the environment, pasteurize foods, and make tools corrosion-resistant.
Future Horizons
Researchers are working on putting plasma to effective usage. Plasma would have to be low energy and ought to be able to endure without immediately reacting and deteriorating. The application of magnetic fields involves the use of plasma.
The magnetic fields create a low-energy plasma that produces molecules that remain in what scientists call a metastable state. The magnetic fields utilized to develop the low-temperature plasma give the plasma molecules, which do not react till they collide with another particle with just the ideal energy. This allows these metastable particles to endure enough time to react with a designated molecule.
These metastable particles are selective in their reactivity. It makes them a possibly unique service to issues like radioactive contamination. Researchers are currently trying out mixtures of gases to work as metastable agents on plutonium and uranium, and this is just the start.
MCQs with Answers
- What is plasma?
- a) The first state of matter
- b) The second state of matter
- c) The third state of matter
- d) The fourth state of matter
- Answer: d
- Who identified plasma in 1879?
- a) William Crookes
- b) Thomas Edison
- c) Nikola Tesla
- d) Marie Curie
- Answer: a
- How is plasma formed?
- a) Cooling of gas
- b) Condensation of liquid
- c) Ionization of gas
- d) Solidification of matter
- Answer: c
- Where is natural plasma found?
- a) In everyday objects
- b) In high-temperature vacuums
- c) In solid structures
- d) In liquids
- Answer: b
- What characteristic defines a plasma?
- a) Macrocopically positive
- b) Macrocopically negative
- c) Macrocopically neutral
- d) Macrocopically solid
- Answer: c
- In which celestial bodies is plasma abundant?
- a) Only in comets
- b) Only in planets
- c) Only in stars
- d) In stars and the sun
- Answer: d
- What is a unique application of plasma in fluorescent light bulbs?
- a) Creating a vacuum
- b) Producing radiation
- c) Charging up the gas
- d) Solidifying matter
- Answer: c
- What happens when an electric current is passed through neon gas in a neon sign?
- a) The gas explodes
- b) It turns into a solid
- c) Plasma and light are produced
- d) Nothing happens
- Answer: c
- What is the primary function of plasma in a fluorescent light bulb?
- a) Generating heat
- b) Producing sound
- c) Creating a vacuum
- d) Producing radiant plasma
- Answer: d
- What is the potential application of plasma in pollution control?
- a) Creating music
- b) Generating electricity
- c) Removal of hazardous chemicals
- d) Solidification of gases
- Answer: c
- What is one future horizon for plasma research?
- a) Creating high-energy plasma
- b) Developing low-energy plasma
- c) Eliminating plasma
- d) Ignoring plasma
- Answer: b
- What is the role of magnetic fields in creating low-temperature plasma?
- a) Heating the plasma
- b) Destroying the plasma
- c) Maintaining the plasma’s metastable state
- d) Making the plasma invisible
- Answer: c
- Why are metastable particles in plasma considered unique in reactivity?
- a) They react immediately
- b) They have high energy
- c) They don’t react until colliding with specific energy
- d) They don’t react at all
- Answer: c
- What makes plasma challenging to use in certain applications?
- a) Its high stability
- b) Its low-energy state
- c) Its rapid reaction with molecules
- d) Its preference for solids
- Answer: c
- What is the focus of current research with plasma and radioactive contamination?
- a) Creating more contamination
- b) Ignoring contamination
- c) Using plasma for contamination removal
- d) Increasing contamination levels
- Answer: c
Summary: Plasma State Tutorial
The tutorial on Plasma State covers the following key aspects:
- Introduction to Plasma:
- Plasma is recognized as the “fourth state of matter,” distinct from solid, liquid, and gas.
- William Crookes identified plasma in 1879.
- Plasmas constitute over 99 percent of the visible universe.
- Formation of Plasma:
- Heat-induced ionization of atoms or molecules leads to the creation of plasma.
- Ionization results in the generation of ions, electrons, and neutral atoms, collectively known as plasma.
- Natural and Artificial Plasma:
- Artificial plasma is produced by ionizing gases, as seen in neon signs.
- Low-temperature plasma is challenging to maintain outside a vacuum due to its rapid reaction with molecules.
- Characteristics of Plasma:
- Plasma requires a significant number of charged particles, responding collectively to electric and magnetic fields.
- Although it conducts electricity, plasma is macroscopically neutral, maintaining an equal balance of electrons and ions.
- Ubiquity of Plasma:
- The universe is predominantly composed of plasma, found from stars to quarks.
- On Earth, plasma is limited to specific regions like lightning bolts, flames, auroras, and fluorescent lights.
- Applications of Plasma:
- Plasma plays a crucial role in various technological applications.
- It is utilized in semiconductors, sanitation of medical products, lamps, lasers, diamond-coated films, and power sources.
- Applications extend to generating electricity from fusion, pollution control, and hazardous chemical removal.
- Diverse Applications:
- Plasma contributes to the functionality of everyday items, from lighting offices and houses to powering computers and electronic devices.
- It drives lasers, particle accelerators, aids in environmental cleanup, pasteurizes foods, and enhances tool corrosion resistance.
- Future Horizons:
- Ongoing research focuses on harnessing plasma for beneficial purposes.
- Low-energy plasma, enduring without rapid degradation, is being explored.
- Magnetic fields play a role in creating low-temperature plasma, leading to molecules in a metastable state.
- Metastable particles in plasma show selective reactivity, holding potential solutions for challenges like radioactive contamination.
In conclusion, the tutorial provides a comprehensive overview of plasma, its formation, characteristics, ubiquity, applications, and future research directions.