Lanthanides and Actinides
F block elements are divided into two series, particularly lanthanoids and actinoids.
- These blocks of elements are typically described as inner transition elements because they provide a transition in the 6th and 7throw of the periodic table which separates the s block and the d block elements.
- We see some hidden “layers” in the periodic table. As we look at the periodic table, we see two boxes– one between Ba (element 56) and Hf (element 72) and the other between Ra (88) and Rf (104).
- These elements all have unfilled f -subshells.
- Due to the uniqueness of the electronic configurations, these elements fit into the two boxes in the larger periodic table.
- As the number of electrons in an atom increases, we begin to see some odd behaviours.
- Due to the way the electron energy levels work, some inner levels fill after several external levels do. We see this in two similar groups of elements– the lanthanides and the actinides.
- F block elements are put separately at the bottom of the periodic table. They are a subset of 6th and 7th periods.
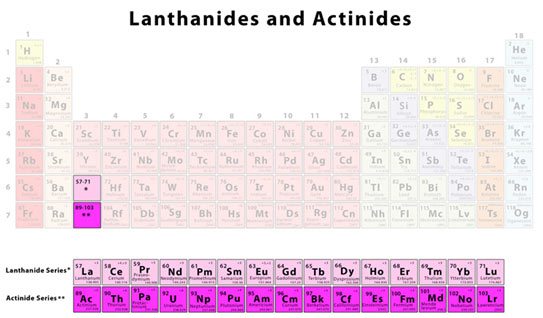
Classification of f- Block Components
The elements coming from the f block are additionally differentiated into:
- The very first series of elements are called lanthanides and include elements with atomic numbers beginning from 57 and ending at 71. These elements are non-radioactive (except for promethium, which is radioactive).
- The second series of elements are called actinides and include elements with atomic numbers beginning from 89 and ending at 103. These elements normally have a radioactive nature.
The row beginning with Lanthanum is the row including all the lanthanides whereas the row beginning with Actinium is the row that contains all the actinides.
Characteristic of Lanthanides
- Lanthanides are soft metals of a silvery-white colour.
- Their colour dulls and their brightness minimizes rapidly when exposed to air.
- They have melting points varying from 1000K to 1200K (Except Samarium, 1623K).
- Lanthanides are excellent conductors of heat and electricity as they have free electrons.
- They are non-radioactive in nature except Promethium.
- A decline in atomic and ionic radii from lanthanum to lutetium is seen. This is called the lanthanoid contraction.
Characteristic of Actinides
- The Actinide elements seem silvery.
- The Actinides have a radioactive nature.
- These metals are extremely reactive and their reactivity increases when they are finely divided.
- A reduction in atomic and ionic radii from Actinium to Lawrencium is seen. This is called the actinoid contraction.
- They typically show an oxidation state of +3. However, elements belonging to the first half of the series are known to display greater oxidation states quite regularly.
Uses of Lanthanides
Lanthanides have been extensively utilized as alloys to impart strength and solidity to metals. The primary lanthanide utilized for this function is cerium, combined with percentages of lanthanum, neodymium, and praseodymium. These metals are likewise extensively utilized in the petroleum industry for refining of petroleum into gasoline items.
Erbium and other lanthanides are commonly used in some optical gadgets, such as night vision safety glasses, laser beams, and phosphorescent products.

Uses of Actinides
The actinides are valuable primarily since they are radioactive. These elements can be used as energy sources for applications as varied as cardiac pacemakers and generation of electrical energy for instruments on the moon. Uranium and plutonium have actually been utilized in nuclear weapons and in nuclear power plants.

Multiple-Choice Questions (MCQs) with Answers:
- What is the unique characteristic of f-block elements?
- a) They are all gases
- b) They provide a transition between s block and d block
- c) They are all non-metals
- d) They have the highest atomic numbers
Answer: b) They provide a transition between s block and d block
- Where are f-block elements located in the periodic table?
- a) In the middle of the periodic table
- b) At the top of the periodic table
- c) At the bottom of the periodic table
- d) Scattered randomly in the periodic table
Answer: c) At the bottom of the periodic table
- Which two series make up the f-block elements?
- a) Lanthanides and Actinides
- b) Alkali metals and Alkaline earth metals
- c) Halogens and Noble gases
- d) Transition metals and Inner transition metals
Answer: a) Lanthanides and Actinides
- Which of the following is a characteristic of lanthanides?
- a) Highly reactive metals
- b) Radioactive in nature
- c) Excellent conductors of electricity
- d) Non-metallic in nature
Answer: c) Excellent conductors of electricity
- What is the lanthanoid contraction?
- a) Increase in atomic and ionic radii
- b) Decrease in atomic and ionic radii
- c) Increase in melting points
- d) Decrease in melting points
Answer: b) Decrease in atomic and ionic radii
- Which element belongs to the actinide series?
- a) Lutetium
- b) Protactinium
- c) Europium
- d) Thallium
Answer: b) Protactinium
- What is a characteristic of actinides?
- a) Non-radioactive nature
- b) High reactivity when finely divided
- c) Silvery color without exception
- d) Lanthanoid contraction
Answer: b) High reactivity when finely divided
- What is the primary use of lanthanides in the petroleum industry?
- a) Catalyzing reactions
- b) Refining petroleum into gasoline products
- c) Producing optical devices
- d) Generating electrical energy
Answer: b) Refining petroleum into gasoline products
- Which lanthanide is commonly used in optical devices like night vision goggles?
- a) Cerium
- b) Erbium
- c) Neodymium
- d) Praseodymium
Answer: b) Erbium
- Why are actinides primarily valuable?
- a) They are all non-radioactive
- b) They are excellent conductors of heat
- c) They can be used in cardiac pacemakers
- d) They are radioactive and useful in various applications
Answer: d) They are radioactive and useful in various applications
- Which actinide element has been used in nuclear weapons and nuclear power plants?
- a) Uranium
- b) Thorium
- c) Protactinium
- d) Americium
Answer: a) Uranium
- What is the actinoid contraction?
- a) Increase in atomic and ionic radii
- b) Decrease in atomic and ionic radii
- c) Increase in reactivity
- d) Decrease in reactivity
Answer: b) Decrease in atomic and ionic radii
FAQs (Frequently Asked Questions):
- What are f-block elements, and why are they called inner transition elements?
- Answer: F-block elements are lanthanides and actinides located at the bottom of the periodic table. They are called inner transition elements because they provide a transition between the s block and the d block in the 6th and 7th rows of the periodic table.
- Why are lanthanides and actinides placed separately at the bottom of the periodic table?
- Answer: Lanthanides and actinides have unfilled f-subshells, and their unique electronic configurations cause them to fit into hidden “layers” at the bottom of the periodic table, separate from the main body.
- What is the lanthanoid contraction?
- Answer: The lanthanoid contraction refers to the decrease in atomic and ionic radii from lanthanum to lutetium among lanthanides. This contraction is observed due to the unique electronic structure of these elements.
- What are the classifications of f-block components?
- Answer: F-block components are classified into two series: lanthanides (atomic numbers 57-71) and actinides (atomic numbers 89-103). Lanthanides are mostly non-radioactive, while actinides are generally radioactive.
- Why are lanthanides called soft metals, and what is the exception?
- Answer: Lanthanides are called soft metals because of their malleability. An exception is samarium, which has a higher melting point of 1623K compared to the typical range of 1000K to 1200K for other lanthanides.
- What is the significance of the actinoid contraction?
- Answer: The actinoid contraction refers to the decrease in atomic and ionic radii from Actinium to Lawrencium among actinides. This contraction is influenced by the unique electronic structure of actinides.
- Why are actinides considered valuable, especially in terms of their radioactive nature?
- Answer: Actinides are valuable due to their radioactive properties. They find applications as energy sources in diverse fields, including cardiac pacemakers, electrical energy generation for moon instruments, and historically in nuclear weapons and power plants.
- How are lanthanides used in the petroleum industry?
- Answer: Lanthanides, especially cerium, are used as alloys to strengthen metals. In the petroleum industry, they play a role in refining petroleum into gasoline products.
- Which optical devices commonly use lanthanides?
- Answer: Lanthanides, such as erbium, are used in optical devices like night vision glasses, laser beams, and phosphorescent products.
- What are some applications of actinides in addition to their radioactive properties?
- Answer: Actinides, besides their radioactive applications, have been historically used in nuclear weapons and power plants. They serve as energy sources for various applications, showcasing their versatility.
- Are lanthanides and actinides typically conductors of heat and electricity?
- Answer: Yes, lanthanides, with free electrons, are excellent conductors of heat and electricity. Actinides also exhibit these properties, contributing to their utility.
- Do lanthanides display any peculiar behavior when exposed to air?
- Answer: Yes, when lanthanides are exposed to air, their color dulls, and brightness reduces rapidly due to specific reactions with atmospheric elements.
Wrap up
This tutorial provides a comprehensive understanding of Lanthanides and Actinides elements in the periodic table. Here’s a summary of the key points covered:
1. F-Block Elements:
- Lanthanides and actinides form the f-block elements, serving as inner transition elements.
- Positioned separately at the bottom of the periodic table, they create a transition between the s block and the d block in the 6th and 7th rows.
2. Hidden Layers in the Periodic Table:
- Two hidden layers exist between specific elements, denoted by boxes containing elements with unfilled f-subshells.
- The electronic configurations of lanthanides and actinides contribute to their unique placement.
3. Classification:
- Lanthanides (atomic numbers 57-71) and actinides (atomic numbers 89-103) are classified into the first and second series, respectively.
- Lanthanides are mostly non-radioactive, except for promethium, while actinides are generally radioactive.
4. Characteristics:
- Lanthanides: Soft, silvery-white metals, good conductors of heat and electricity, with a lanthanoid contraction.
- Actinides: Silvery, radioactive metals, reactive, with an actinoid contraction and variable oxidation states.
5. Uses:
- Lanthanides: Utilized as alloys (e.g., cerium) for strength, in the petroleum industry, and in optical devices (e.g., erbium).
- Actinides: Valuable for their radioactive properties, used in energy sources (e.g., pacemakers, moon instruments) and historically in nuclear weapons and power plants.
6. Conclusion:
- Lanthanides and actinides play crucial roles in various industries, from strengthening metals to serving as essential components in optical and energy-related applications. Understanding their unique characteristics is vital for appreciating their diverse applications in science and technology.

