Isomerism and its Types
In organic chemistry, isomers are particles with the very same molecular formula (i.e., the same number of atoms of each element), but different structural or spatial arrangements of the atoms within the molecule. The reason there is such an enormous variety of organic compounds– more than 10 million– remains in part down to isomerism.
Definition of Isomerism
Isomerism is the phenomenon in which more than one compounds have the same chemical formula however various chemical structures. Chemical compounds that have similar chemical formulae but differ in properties and the plan of arrangement of the atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are called isomers.
The word “isomer” is originated from the Greek words “isos” and “meros”, which mean “equal parts”. This term was coined by the Swedish chemist Jacob Berzelius in the year 1830.
Types of Isomerism
Generally, there are 2 types. They are:
- Structural Isomerism
- Stereoisomerism
However, these have again many subtypes.
Structural Isomerism
Isomers are structural isomers when they have the very same molecular formula however different structures. Structural isomerism is even more of the following types. Let’s learn more about these types.
1) Chain Isomerism
Isomers are chain isomers when 2 or more compounds have the exact same molecular formula however vary in the branching of carbon atoms.

2) Position isomerism
Isomers are position isomers when the two or more compounds differ in the position of the functional group or substituent atoms.
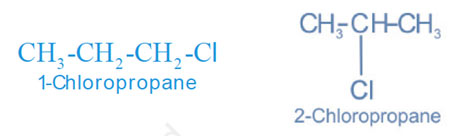
3) Functional Isomerism
Isomers are functional isomers when the two or more compounds have an identical molecular formula but vary in the functional group present.

4) Metamerism
This is shown by compounds due to the existence of different alkyl chains on either side of the functional group.

Stereo Isomerism
Stereoisomerism is a phenomenon in which compounds have the same molecular formula however vary in the relative positioning or orientation of atoms in space. Stereoisomers are the compounds displaying stereoisomerism. We can, even more, categorize stereoisomerism into:
(a) Geometrical isomerism:
The isomers which have the same structural formula however differ in the spatial arrangement of the groups around the double bond are called geometrical isomers and the phenomenon is referred to as geometrical isomerism. This isomerism is shown by alkenes or their derivatives. In cis-isomer, similar groups reside on the same side, while the similar groups when lying on opposite sides, the isomer is referred to as trans.

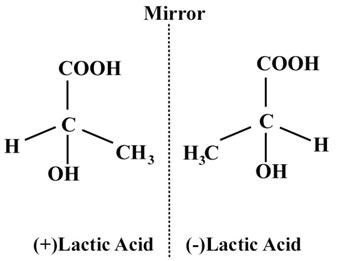
(b) Optical isomerism:
This kind of isomerism occurs from various plans of the arrangement of atoms or groups in space, leading to two isomers which are the mirror images of each other. Optical isomers include an asymmetric (chiral) carbon atom (a carbon atom attached to four different atoms or groups) in their molecules. Optical isomers have similar chemical and physical properties and vary only in their behavior towards plane-polarized light.
The isomer which turns the plane-polarized light to left is called laevo (1) while that rotates the plane-polarized light to the right is called Dextro (d).
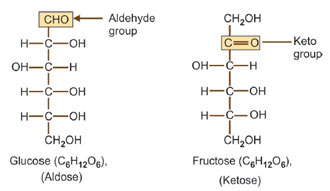
Other Isomerism displayed by sugars
Ketose-Aldose isomerism
Glucose and fructose are isomers of each other having the same chemical (molecular) formula C6H12O6, however, they differ in structural formula with respect to their D and L isomers are mirror images of each other. These two kinds are called enantiomers. Most of the monosaccharides in living beings belong to the D-series.
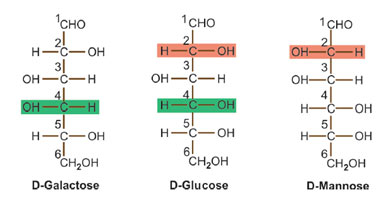
Epimerism
When two monosaccharides differ from each other in their configuration around a single uneven carbon (aside from anomeric carbon) atom, they are referred to as epimers of each other.
For instance, galactose and mannose are 2 epimers of glucose. They vary from glucose in the configuration of groups (H and OH) around C-4 and C-2 respectively. Galactose and mannose are not epimers of each other as they vary in configuration at 2 asymmetric carbon atoms around C-2 and C-4.
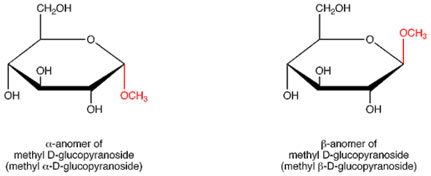
Anomerism
α and β Anomerism
The primary type of glucose and fructose in a solution is not an open chain. Rather, the open-chain form of these sugar in solution cyclize into rings. An extra uneven center is created when glucose cyclizes. Carbon-1 of glucose in the open-chain type ends up being an uneven carbon in the ring type and 2 ring structures can be formed. These are:
- – α-D-glucose
- – β-D-glucose
The classification α implies that the hydroxyl group attached to C-1 is below the plane of the ring, β implies that it is above the plane of the ring. The C-1 carbon is called the anomeric carbon atom and so, α and β types are anomers.
MCQs about Isomerism
- What is isomerism in organic chemistry?
- A) The variation in molecular formulas
- B) The phenomenon of compounds with the same formula but different structures
- C) The presence of different functional groups
- D) The arrangement of atoms in a molecule
Answer: B
- Who coined the term “isomer” in the year 1830?
- A) Robert Boyle
- B) Antoine Lavoisier
- C) Jacob Berzelius
- D) Linus Pauling
Answer: C
- How many main types of isomerism are generally recognized?
- A) 1
- B) 2
- C) 3
- D) 4
Answer: B
- Structural isomerism involves isomers with:
- A) Same molecular formula and structure
- B) Same molecular formula but different structures
- C) Different molecular formulas
- D) Different functional groups
Answer: B
- Chain isomerism is characterized by differences in:
- A) Functional groups
- B) Molecular formulas
- C) Carbon atom branching
- D) Stereoisomerism
Answer: C
- Position isomerism refers to differences in the:
- A) Molecular weight
- B) Position of the functional group or substituent atoms
- C) Optical activity
- D) Isotope distribution
Answer: B
- Functional isomerism is observed when compounds have:
- A) Different molecular weights
- B) Identical molecular formulas but different functional groups
- C) Opposite stereochemistry
- D) Identical structures
Answer: B
- Metamerism is a result of:
- A) Different alkyl chains on either side of the functional group
- B) Identical alkyl chains
- C) Same molecular weight
- D) Geometrical arrangement of atoms
Answer: A
- Stereoisomerism involves differences in:
- A) Molecular weight
- B) Molecular formula
- C) Spatial arrangement of atoms in space
- D) Functional groups
Answer: C
- Geometrical isomerism is exhibited by:
- A) Alkanes
- B) Aldehydes
- C) Alkenes or their derivatives
- D) Aromatic compounds
Answer: C
- In geometrical isomerism, what is the term used for similar groups residing on opposite sides?
- A) Cis
- B) Trans
- C) Iso
- D) Meta
Answer: B
- Optical isomerism results from:
- A) Different molecular weights
- B) Different plans of atom arrangement
- C) Identical stereochemistry
- D) Same chirality
Answer: B
- How are optical isomers named based on their rotation of plane-polarized light?
- A) Dextro and Levorotary
- B) Alpha and Beta
- C) Cis and Trans
- D) Anomers
Answer: A
- Which type of isomerism is displayed by glucose and fructose?
- A) Geometrical isomerism
- B) Ketose-Aldose isomerism
- C) Epimerism
- D) Anomerism
Answer: B
- What are glucose and fructose considered as in terms of isomerism?
- A) Enantiomers
- B) Diastereomers
- C) Constitutional isomers
- D) Structural isomers
Answer: A
- Epimerism is characterized by differences in configuration around:
- A) An asymmetric carbon
- B) An alkyl group
- C) The functional group
- D) The molecular center
Answer: A
- What is formed when glucose cyclizes in solution?
- A) Open-chain structure
- B) Linear structure
- C) Ring structure
- D) Branched structure
Answer: C
- What is the extra asymmetric center created when glucose cyclizes?
- A) C-2
- B) C-4
- C) Anomeric carbon (C-1)
- D) C-6
Answer: C
- What do α and β in anomeric carbon isomers signify?
- A) Atomic mass
- B) Stereochemistry
- C) Optical activity
- D) Isotope distribution
Answer: B
- Which isomer rotates plane-polarized light to the left?
- A) Alpha
- B) Beta
- C) Dextro
- D) Levorotary
Answer: D
- What is the term used for the phenomenon where the open-chain form of sugars cyclizes into rings?
- A) Ring isomerism
- B) Anomerism
- C) Chain isomerism
- D) Structural isomerism
Answer: B
Frequently Asked Questions (FAQs) – Isomerism
1. What is isomerism in organic chemistry?
- Isomerism in organic chemistry refers to the phenomenon where compounds share the same molecular formula but have different structural or spatial arrangements of atoms within the molecule.
2. Who coined the term “isomer” and when?
- The term “isomer” was coined by the Swedish chemist Jacob Berzelius in the year 1830.
3. How many types of isomerism are there?
- There are two main types of isomerism: structural isomerism and stereoisomerism.
4. What is the significance of the word “isomer”?
- The word “isomer” is derived from the Greek words “isos” and “meros,” meaning “equal parts.”
5. What are structural isomers?
- Structural isomers are compounds that share the same molecular formula but have different structures. This type of isomerism includes chain isomerism, position isomerism, functional isomerism, and metamerism.
6. What is chain isomerism?
- Chain isomerism occurs when two or more compounds have the same molecular formula but differ in the branching of carbon atoms.
7. Explain position isomerism.
- Position isomerism happens when two or more compounds differ in the position of the functional group or substituent atoms.
8. What is stereoisomerism?
- Stereoisomerism is a phenomenon where compounds share the same molecular formula but differ in the relative positioning or orientation of atoms in space. It includes geometrical isomerism and optical isomerism.
9. What is geometrical isomerism?
- Geometrical isomerism occurs when isomers have the same structural formula but differ in the spatial arrangement of groups around a double bond. It is shown by alkenes or their derivatives.
10. How is optical isomerism defined?
- Optical isomerism arises from different plans of the arrangement of atoms or groups in space, resulting in two mirror-image isomers. It involves an asymmetric (chiral) carbon atom in the molecules.
11. Provide an example of sugars displaying isomerism.
- Glucose and fructose are isomers with the same chemical formula C6H12O6 but differ in structural formula, exhibiting ketose-aldose isomerism.
12. What is epimerism?
- Epimerism occurs when two monosaccharides differ in configuration around a single uneven carbon (except for the anomeric carbon) atom.
13. Explain anomerism in sugars.
- Anomerism refers to the existence of α and β anomers when open-chain sugars cyclize into rings. They represent different spatial arrangements of the hydroxyl group at the anomeric carbon.
14. How are α-D-glucose and β-D-glucose classified?
- α-D-glucose has the hydroxyl group at C-1 below the plane of the ring, while β-D-glucose has it above the plane. They are termed anomers.
15. Why is isomerism essential in organic compounds?
- Isomerism contributes to the vast variety of organic compounds, enabling more than 10 million different structures with the same molecular formula.
16. Are all monosaccharides in living beings part of the D-series?
- Yes, most monosaccharides in living beings belong to the D-series.
17. How does stereoisomerism affect the properties of compounds?
- Stereoisomers have similar chemical and physical properties but may differ in their behavior towards plane-polarized light or other chiral interactions.
18. Can a compound exhibit both structural and stereoisomerism simultaneously?
- Yes, a compound can exhibit both structural and stereoisomerism, providing a diverse range of isomeric possibilities.
Summary
The tutorial on Isomerism provides a comprehensive exploration of the concept, its types, and specific examples, particularly focusing on structural and stereoisomerism. Here’s a brief summary of the key points covered:
1. Introduction to Isomerism:
- Isomers are molecules with the same molecular formula but different structural or spatial arrangements of atoms.
- Over 10 million organic compounds exist, largely due to isomerism.
2. Definition of Isomerism:
- Isomerism is the phenomenon where multiple compounds share the same chemical formula but have different chemical structures.
3. Types of Isomerism:
- Two main types: Structural Isomerism and Stereoisomerism.
4. Structural Isomerism:
- 4.1 Chain Isomerism: Same molecular formula, differing in the branching of carbon atoms.
- 4.2 Position Isomerism: Differ in the position of functional groups or substituent atoms.
- 4.3 Functional Isomerism: Identical molecular formula but vary in the functional group.
- 4.4 Metamerism: Occurs due to different alkyl chains on either side of the functional group.
5. Stereoisomerism:
-
- Phenomenon where compounds have the same molecular formula but differ in the spatial arrangement of atoms.
- 5.1 Geometrical Isomerism: Groups around a double bond differ in spatial arrangement, shown by alkenes.
- 5.2 Optical Isomerism: Mirror-image isomers due to asymmetric (chiral) carbon atoms. Laevo and Dextro isomers rotate plane-polarized light to the left and right, respectively.
6. Other Isomerism Displayed by Sugars:
- 6.1 Ketose-Aldose Isomerism: Glucose and fructose are enantiomers, belonging to the D-series in living beings.
- 6.2 Epimerism: Two monosaccharides differing in configuration around a single uneven carbon atom.
- 6.3 Anomerism: α and β anomers formed when open-chain sugars cyclize into rings.
In summary, this tutorial serves as a comprehensive guide to understanding isomerism, offering insights into its various types and real-world applications, particularly in organic chemistry.

