Definition:
Hemoglobin is conjugated proteins, with a prosthetic group heme. Hemoglobin found in red blood cells carries oxygen from the lungs to the cells as well as carbon dioxide from tissues to the lungs. The hemoglobin content of erythrocytes contributes red color to blood.
Structure:
Hemoglobin is a globular, oligomeric protein comprised of heme and globin groups.
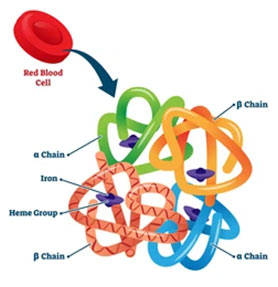
Heme + globin → Hemoglobin
- Heme
The heme is the iron-containing part that belongs to the class of compounds called protoporphyrin. Protoporphyrin is made up of four pyrrole rings which are linked by a methene (= CH) bridge to form a porphyrin ring. 4 methyl, 2 vinyl, and two propionate side chain groups are connected to the porphyrin ring. These substituents can be arranged in 15 different methods.
But only one of these isomers called protoporphyrin IX is naturally active. The iron (Fe2+) is held in the center of the proto-porphyrin molecule by coordination bonds with the 4 nitrogen atoms of the protoporphyrin ring.
The iron atom has six coordination bonds. 4 bonds are created between the iron and nitrogen atoms of the porphyrin ring system. 5th bond is developed in between nitrogen atom of histidine residue of the globin polypeptide chain, called proximal histidine. The sixth bond is formed with oxygen. The oxygenated form of hemoglobin is stabilized by the H-bond between oxygen as well as the side chain of another histidine residue of the globin chain, known as distal histidine.
- Globin
Globin molecule includes four polypeptide chains, 2 alpha (α) chains (141 amino acid residues each) as well as two beta (β) or two gammas (γ) or two deltas (δ) as per the kind of hemoglobin. The β, γ, as well as δ chains have 146 amino acid residues each. With each polypeptide chain, one molecule of heme is attached. A hemoglobin molecule, therefore has 4 heme molecules. 4 globin polypeptide chains with 4 heme are held together in a definite arrangement or conformation to constitute a characteristic quaternary structure of hemoglobin, which is stabilized by:
- Hydrogen bonds
- Salt bridges
- Van der Waals forces
There is little contact between two alpha and two beta chains. But there are several contact factors between alpha and also beta chains of dissimilar chain pairs α1β1 and α2β2. There is a central open network or cavity in the hemoglobin molecule. The function of the globin chain of hemoglobin is to create a protective hydrophobic pocket for binding of heme. These pockets protect the reduced form of iron (Fe2+) of heme from oxidizing to the ferric (Fe3+) form, from the aqueous environment and also permit binding of oxygen with Fe2+ion of heme. Direct exposure of heme iron to water causes oxidation of Fe2+to Fe3+ kind and loss of oxygen binding capability.
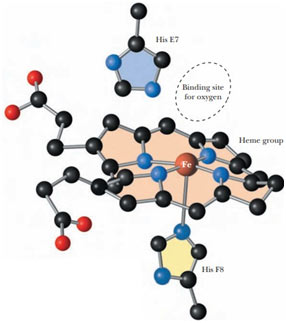
Binding sites on Hemoglobin
- Oxygen
Oxygen is bound to the ferrous (Fe2+) atom of the heme to create oxyhemoglobin.
- Hydrogen
Hydrogen is bound to R-groups (side chain) of histidine residues of α and β chains.
- Carbon dioxide
Carbon dioxide is bound to the N-terminal end of each of the polypeptide chains of hemoglobin to form carbamino hemoglobin.
Types of Hemoglobin:
Adult Hemoglobin (HbA1)
It contains two alpha and two beta chains and is designated as α2β2. Around 98 percent of the overall hemoglobin of a normal adult is of this kind.
Minor Component of NormalAdult Hemoglobin (HbA2)
It contains two alpha as well as two delta chains and is designated as α2δ2. It is present typically for 2.5 percent of the total amount.
Fetal Hemoglobin (HbF)
HbF is the significant hemoglobin found in a fetus or newborn or infant. It contains two alpha as well as 2 gamma chains. After birth, the gamma (γ) subunits are changed by beta (β) chains and it becomes α2β2 (HbA1).
Glycosylated Hemoglobin (HbA1C)
HbA1 (adult Hb) reacts spontaneously with glucose to form a by-product, referred to as glycosylated hemoglobin (HbA1C). Measurement of HbA1C gives helpful details.
For the monitoring of diabetes mellitus, since RBCs have a lifetime of around 120 days, the content of HbA1C is a sign of how successfully blood sugar levels have actually been regulated over the previous 2 to 3 months.
Functions of Hemoglobin
- Transportation of O2 from lungs to tissues.
- Transport of CO2 and also H+ from tissues to lungs as well as kidney.
- Serve as an intracellular buffer and is hence associated with acid-base equilibrium.