- 1) Dalton’s Law of Partial Pressures
- 2) Estimation of Partial Pressure of a Gas
- 3) Applications of Dalton’s Law of Partial Pressures
- 4) MCQs on Dalton’s Law of Partial Pressures
- 5) Frequently Asked Questions (FAQs) – Dalton’s Law of Partial Pressures
- 6) Problem/Solutions
- 7) Summary
- 8) You may also like to learn:
Dalton’s Law of Partial Pressures
John Dalton studied the mixtures of gases and offered his law of partial pressures. According to this law,
“The total pressure exerted by a mixture of non-reacting gases is equal to the amount of their partial pressures”.
Let the gases be designated as 1,2,3, and their partial pressures are p1, p2, p3. The total pressure (P) of the mixture of gases is given by
Pt = p1+ p2 + p3
The partial pressure of a gas in a mix of gases is the pressure that it would exert on the walls of the container if it were present all alone in that same volume under the same temperature.
Let us have 4 cylinders of the same volume, i.e., 10 dm3 each and three gases H2, CH4 and O2 are independently confined in the first three of them at the same temperature level. Let their partial pressures be 400 torr, 500 torr and 100 torr, respectively. All these gases are transferred to the 4th cylinder of capacity 10 dm3 at the same temperature level. According to Dalton’s law
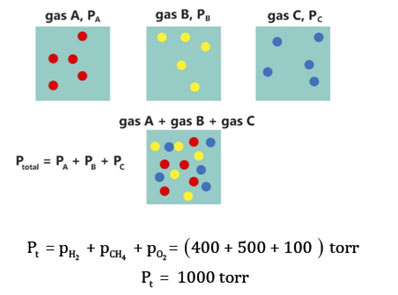
These 3 non-reacting gases are behaving independently under regular conditions. The rapidly moving molecules of each gas in a mixture have equal opportunity fields to hit the walls of the container. Thus, each gas applies a pressure independent of the pressure of other gases. The total pressure is the outcome of an overall number of collisions per unit area in a provided time.
Particles of each gas move individually, so the general gas equation (PV = nRT) can be applied to the individual gases in the gaseous mixture.
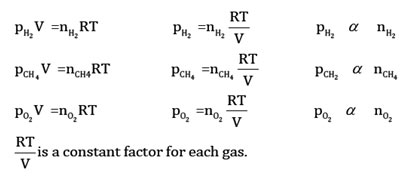
All these gases have their partial pressures. Because volumes and temperature levels are the same, so their number of moles will be different and will be directly proportional to their partial pressures. Adding these three equations
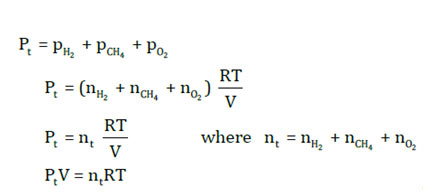
The total pressure of the mix of gases relies on the total number of moles of the gases.
Estimation of Partial Pressure of a Gas
The partial pressure of any gas in a mix of gases can be determined, provided one knows the mass of that gas or its number of moles along with the total pressure and the overall number of moles present in the mixture. To have a relationship, let us suppose that we have a mixture of gas A and gas B.
This mixture is enclosed in a container having volume (V). The overall pressure is one atm. The number of moles of the gases A and B is nA and nB respectively. If they are kept at temperature level T, then
P V = n RT(equation for the mixture of gases)
p V = n RT(equation for gas A)
p V = n RT(equation for gas B)
Divide the first two equations
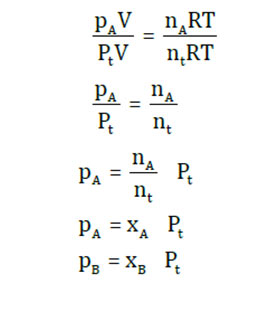
Similarly,
The partial pressure of a gas is the mole fraction of that gas increased by the overall pressure of the mixture. Bear in mind that the mole fraction of any of the gases in the mix is less than unity. Furthermore, the amount of mole fraction is constantly equal to unity.
Applications of Dalton’s Law of Partial Pressures
Following are the four crucial applications of Dalton’s Law of partial pressures.
- Some gases are gathered over water in the laboratory. The gas throughout the collection collects water vapours and becomes moist. The pressure exerted by this moist gas is, for that reason, the sum of the partial pressures of the dry gas and that of water vapours.
The partial pressure put in by the water vapours is called aqueous tension.
Pmoist = pdry + pw.vap
Pmoist = pdry+ aqueous tension
p dry= P moist– aqueous tension
While resolving the numerical the aqueous tension is subtracted from the total pressure (P moist).
- Dalton’s law finds its applications during the process of respiration. The process of respiration depends upon the difference in partial pressures. When animals inhale air then oxygen moves into the lungs as the partial pressure of oxygen in the air is 159 torr, while the partial pressure of oxygen in the lungs 116 torr. CO2 produced throughout respiration leaves in the opposite direction, as its partial pressure is higher in the lungs than in air.
- At higher elevations, the pilots feel uncomfortable breathing since the partial pressure of oxygen in the un-pressurized cabin is low, as compared to 159 torr, where one feels comfortable breathing.
- Deep-sea divers take oxygen blended with an inert gas to say He and change the partial pressure of oxygen according to the requirement. In fact, in the sea after every 100 feet depth, the diver experiences approximately 3 atm pressure, so normal air cannot be inhaled depth of the sea. Moreover, the pressure of N2 boosts the depth of the sea and it diffuses in the blood.
MCQs on Dalton’s Law of Partial Pressures
- What did John Dalton study to propose his law of partial pressures?
- a) Liquid Mixtures
- b) Gaseous Mixtures
- c) Solid Mixtures
- d) Aqueous Mixtures
- Answer: b
- According to Dalton’s Law, what is the total pressure of a mixture of non-reacting gases equal to?
- a) Sum of their individual temperatures
- b) Sum of their individual masses
- c) Sum of their individual partial pressures
- d) Sum of their individual volumes
- Answer: c
- If the partial pressures of gases A, B, and C are p1, p2, and p3, what is the total pressure (Pt) of the gas mixture according to Dalton’s Law?
- a) Pt = p1 * p2 * p3
- b) Pt = p1 – p2 – p3
- c) Pt = p1 + p2 + p3
- d) Pt = p1 / p2 / p3
- Answer: c
- How does Dalton’s Law apply to a mixture of gases confined in separate cylinders at the same temperature?
- a) The gases exert equal pressures, irrespective of the cylinder volume.
- b) The total pressure is the sum of individual pressures in each cylinder.
- c) The gas with the highest pressure dominates the total pressure.
- d) Each gas exerts pressure independently of the others.
- Answer: d
- What is the mole fraction of a gas in a mixture equal to?
- a) Total pressure of the mixture
- b) Partial pressure of the gas
- c) Sum of the individual moles of gases
- d) Ratio of the moles of that gas to the total moles of gases
- Answer: d
- How is the partial pressure of a gas calculated if its mass and the total number of moles in the mixture are known?
- a) Multiplying its mass by the total pressure
- b) Dividing its mass by the total pressure
- c) Multiplying its mole fraction by the total pressure
- d) Dividing its mole fraction by the total pressure
- Answer: c
- Which application involves the subtraction of aqueous tension from the total pressure in the laboratory?
- a) Deep-sea diving
- b) Animal respiration
- c) Gas collection over water
- d) Pilot discomfort at high elevations
- Answer: c
- Why do pilots feel uncomfortable breathing at higher elevations according to Dalton’s Law?
- a) Low atmospheric pressure
- b) High partial pressure of oxygen
- c) Low partial pressure of oxygen
- d) High partial pressure of nitrogen
- Answer: c
- What is the crucial factor influencing the process of respiration according to Dalton’s Law?
- a) Difference in partial pressures
- b) Humidity in the air
- c) Volume of gases
- d) Total pressure of inhaled air
- Answer: a
- Why do deep-sea divers use oxygen blended with inert gases during their dives?
- a) To increase the partial pressure of oxygen
- b) To decrease the total pressure underwater
- c) To counteract the effects of nitrogen pressure
- d) To enhance the buoyancy underwater
- Answer: c
- What is the formula for the total pressure (Pt) in a gas mixture according to Dalton’s Law?
- a) Pt = p1 * p2 * p3
- b) Pt = p1 – p2 – p3
- c) Pt = p1 + p2 + p3
- d) Pt = p1 / p2 / p3
- Answer: c
- In the gaseous mixture equation PV = nRT, how do the individual gases behave under normal conditions?
- a) They share a common pressure
- b) Their pressures are dependent on the total pressure
- c) Each gas applies pressure independently
- d) They have no effect on the total pressure
- Answer: c
- How does Dalton’s Law apply to the estimation of the partial pressure of a gas in a mixture?
- a) It involves the mass and temperature of the gas.
- b) It considers the volume and pressure of the gas.
- c) It relies on the mole fraction and total pressure of the mixture.
- d) It is based on the density and composition of the gas.
- Answer: c
- What does the mole fraction of a gas in a mixture represent?
- a) The ratio of its pressure to the total pressure
- b) The number of moles of that gas to the total moles of gases
- c) The total volume occupied by the gas
- d) The product of its mass and the total mass of gases
- Answer: b
- In Dalton’s Law, how are the partial pressures of gases affected when confined in separate containers?
- a) They remain constant
- b) They add up to the total pressure
- c) They compete for dominance
- d) They decrease proportionally
- Answer: a
- What is the relationship between the mole fraction of a gas and its partial pressure?
- a) Directly proportional
- b) Inversely proportional
- c) Exponential relationship
- d) No relationship
- Answer: a
- Which gas law equation is utilized to estimate the partial pressure of a gas in a mixture?
- a) Boyle’s Law
- b) Charles’s Law
- c) Avogadro’s Law
- d) Ideal Gas Law
- Answer: d
- What factor is responsible for the discomfort felt by pilots at higher elevations?
- a) Low total pressure
- b) High partial pressure of oxygen
- c) Low partial pressure of oxygen
- d) High partial pressure of nitrogen
- Answer: c
- What is the significance of the difference in partial pressures in the process of respiration?
- a) Regulates the volume of inhaled air
- b) Controls the temperature of inhaled air
- c) Determines the rate of respiration
- d) Influences the humidity of inhaled air
- Answer: c
- How is the total pressure of a gas mixture affected by the number of moles of gases present?
- a) It remains constant
- b) It decreases proportionally
- c) It increases proportionally
- d) It varies unpredictably
- Answer: c
- What role does Dalton’s Law play in the process of gas collection over water?
- a) It regulates the pressure of collected gases
- b) It accounts for the presence of water vapors in the collected gas
- c) It determines the humidity of the collected gas
- d) It ensures the purity of the collected gases
- Answer: b
- How does Dalton’s Law apply to deep-sea diving practices?
- a) It controls the buoyancy of the diver
- b) It regulates the pressure of inhaled air
- c) It influences the partial pressure of oxygen underwater
- d) It determines the temperature of underwater air
- Answer: c
- What gas-related challenge do deep-sea divers face with increasing depth in the sea?
- a) Decreased total pressure
- b) Increased partial pressure of oxygen
- c) Enhanced buoyancy
- d) Elevated pressure of nitrogen
- Answer: d
Frequently Asked Questions (FAQs) – Dalton’s Law of Partial Pressures
- What is Dalton’s Law of Partial Pressures?
- Dalton’s Law states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of their individual partial pressures.
- How is the total pressure (Pt) of a gas mixture calculated according to Dalton’s Law?
- The total pressure is given by the sum of the partial pressures of the individual gases: Pt = p1 + p2 + p3.
- What is the significance of partial pressure in a mixture of gases?
- The partial pressure of a gas in a mixture represents the pressure it would exert if it occupied the entire volume alone under the same temperature.
- Illustrate Dalton’s Law with an example of three gases in separate cylinders.
- Imagine three cylinders with gases H2, CH4, and O2. When transferred to a fourth cylinder, their individual pressures contribute independently, following Dalton’s Law.
- How do rapidly moving gas molecules behave in a mixture, according to Dalton’s Law?
- In a mixture, rapidly moving gas molecules behave independently, exerting pressure on the container walls without influencing each other’s pressures.
- How is the general gas equation (PV = nRT) applied to individual gases in a mixture?
- Each gas in the mixture follows the general gas equation independently, considering its own partial pressure, volume, moles, and temperature.
- How is the total pressure affected by the number of moles of gases in the mixture?
- The total pressure of the gas mixture depends on the total number of moles of gases present, as each gas contributes to the overall pressure.
- Explain the estimation of the partial pressure of a gas in a mixture.
- The partial pressure of a gas can be determined by knowing its mass or moles, along with the total pressure and the overall number of moles in the mixture.
- What is the mole fraction, and how does it relate to the partial pressure of a gas?
- The mole fraction of a gas is its moles divided by the total moles. The partial pressure of a gas is the mole fraction multiplied by the overall pressure.
- What are some applications of Dalton’s Law of Partial Pressures?
- Applications include gas collection over water, understanding respiration, explaining discomfort at higher elevations, and addressing challenges faced by deep-sea divers.
- Explain the concept of aqueous tension and its relevance in Dalton’s Law.
- Aqueous tension is the partial pressure exerted by water vapors in a moist gas. It is subtracted from the total pressure to determine the pressure of the dry gas.
- How does Dalton’s Law relate to the process of respiration?
- Respiration relies on the difference in partial pressures. Oxygen moves into the lungs during inhalation, where its partial pressure differs from that in the inhaled air.
- Why do pilots feel uncomfortable breathing at higher elevations?
- Pilots feel discomfort due to the low partial pressure of oxygen in un-pressurized cabins at higher elevations, making it challenging to breathe comfortably.
- What challenges do deep-sea divers face, and how does Dalton’s Law explain them?
- Deep-sea divers face challenges due to increased partial pressure of nitrogen at depth. Dalton’s Law helps explain the need for adjusting the partial pressure of oxygen during dives.
- How is Dalton’s Law applied in gas collection over water in the laboratory?
- In gas collection over water, the pressure exerted by the moist gas is the sum of the partial pressures of the dry gas and the aqueous tension.
- In what scenarios does Dalton’s Law play a role in adjusting the partial pressure of oxygen?
- Dalton’s Law is applied in scenarios such as deep-sea diving, where divers use oxygen blended with inert gases to adjust the partial pressure of oxygen based on depth.
- How does Dalton’s Law explain the discomfort experienced by pilots at higher elevations?
- Dalton’s Law attributes the discomfort to the low partial pressure of oxygen in un-pressurized cabins at higher elevations compared to the comfortable breathing level of 159 torr.
- What is the significance of the difference in partial pressures in the process of respiration?
- The difference in partial pressures during respiration determines the rate of respiration, allowing the exchange of gases like oxygen and carbon dioxide in the lungs.
Problem/Solutions
- Problem: Three non-reacting gases, A, B, and C, are present in a container with partial pressures of 200 kPa, 300 kPa, and 150 kPa, respectively. Calculate the total pressure exerted by the mixture.
- Solution: P_total = P_A + P_B + P_C = 200 kPa + 300 kPa + 150 kPa = 650 kPa.
- Problem: A gas mixture contains nitrogen (N2) and oxygen (O2) with partial pressures of 400 mmHg and 300 mmHg, respectively. Determine the total pressure of the mixture.
- Solution: P_total = P_N2 + P_O2 = 400 mmHg + 300 mmHg = 700 mmHg.
- Problem: If a gas has a partial pressure of 800 Pa in a mixture with a total pressure of 1200 Pa, find the partial pressures of the other gases if there are two more gases in the mixture.
- Solution: Let P_A = 800 Pa, and the total pressure P_total = 1200 Pa. The sum of the other partial pressures is P_B + P_C = P_total – P_A = 1200 Pa – 800 Pa = 400 Pa.
- Problem: A mixture of gases at a total pressure of 2 atm contains hydrogen (H2) and helium (He) with partial pressures of 0.8 atm and 0.5 atm, respectively. Find the partial pressure of each gas.
- Solution: P_H2 = 0.8 atm, P_He = 0.5 atm.
- Problem: Three cylinders each contain a different gas at the same temperature. If the partial pressures of gases in the first two cylinders are 300 kPa and 400 kPa, what is the total pressure when the gases are combined into a third cylinder?
- Solution: P_total = P_1 + P_2 = 300 kPa + 400 kPa = 700 kPa.
- Problem: A mixture of gases has partial pressures of 600 torr, 800 torr, and 200 torr for gases X, Y, and Z, respectively. Calculate the total pressure of the mixture.
- Solution: P_total = P_X + P_Y + P_Z = 600 torr + 800 torr + 200 torr = 1600 torr.
- Problem: In a gas mixture, the partial pressure of carbon dioxide (CO2) is 250 mmHg, and the partial pressure of oxygen (O2) is 150 mmHg. Determine the total pressure of the gas mixture.
- Solution: P_total = P_CO2 + P_O2 = 250 mmHg + 150 mmHg = 400 mmHg.
- Problem: A gas mixture consists of methane (CH4) and ethane (C2H6) with partial pressures of 300 kPa and 200 kPa, respectively. Find the total pressure of the mixture.
- Solution: P_total = P_CH4 + P_C2H6 = 300 kPa + 200 kPa = 500 kPa.
- Problem: If the partial pressure of nitrogen in a gas mixture is 600 Pa and the total pressure is 800 Pa, find the partial pressure of the other gases in the mixture.
- Solution: Let P_N2 = 600 Pa, and the sum of the partial pressures of other gases is P_total – P_N2 = 800 Pa – 600 Pa = 200 Pa.
- Problem: A gas mixture contains helium (He) and neon (Ne) with partial pressures of 0.6 atm and 0.4 atm, respectively. Determine the total pressure of the mixture.
- Solution: P_total = P_He + P_Ne = 0.6 atm + 0.4 atm = 1.0 atm.
Summary
In this tutorial on Dalton’s Law of Partial Pressures, we learned that the total pressure exerted by a mixture of non-reacting gases is the sum of their individual partial pressures. John Dalton’s law is expressed as , where each is the partial pressure of a specific gas. Each gas in a mixture behaves independently, exerting pressure as if it were alone in the same volume and under the same temperature.
The estimation of partial pressure involves knowing the mass or number of moles of a gas, the total pressure, and the total number of moles in the mixture. The partial pressure of a gas (�) is calculated as the mole fraction of that gas multiplied by the total pressure of the mixture.
Applications of Dalton’s Law include understanding the behavior of gases collected over water, where the total pressure is the sum of the partial pressures of the dry gas and water vapors. In respiratory processes, Dalton’s Law explains the exchange of gases in the lungs based on differences in partial pressures. Additionally, it has implications for situations such as high-altitude flying discomfort and deep-sea diving, where adjusting the partial pressure of oxygen is crucial for safety and comfort.
