Overview
The technique used in the laboratory for the separation of mixtures is called chromatography. The mixtures are usually dissolved in a fluid solvent like gas or liquid.
This technique has two phases. The stationary phase is one that is fixed and the mobile phase which moves over stationary phase and maybe gas or liquid.
The chromatography having solid stationary phase is adsorption chromatography and one that has a liquid stationary phase is partition chromatography.
For accurate and error-free analysis of food, drugs, chemicals, water samples chromatography is very helpful.
Chromatography
The word chromatography stems from the Greek word “Khromatos” meaning colour writing.
Chromatography is a technique utilized mostly for the separation of a sample of the mixture. It includes the distribution of a solute in between a stationary phase and a mobile phase. The fixed phase might be a solid or a liquid supported as a thin film on the surface of an inert solid. The mobile phase streaming over the surface of the fixed phase may be a gas or a liquid.
In chromatography, compounds are separated due to their relative affinities for the fixed and mobile phases. The distribution of the elements of a mix between the two phases is governed by circulation coefficient K.
K = Concentration of a component in the moving phase/ Concentration of that element in the Fixed phase
The part of a mixture with a small value of K primarily stays in the stationary phase as the moving phase streams over it. The part with a higher value of K remains largely dissolved in the mobile phase and passes over the fixed phase rapidly. Chromatography in which the stationary phase is solid is classified as adsorption chromatography. In this type, a substance leaves the mobile phase to end up being adsorbed on the surface of the solid phase. Chromatography in which the fixed phase is a liquid is called partition chromatography. In this type, the substances being separated are distributed throughout both the fixed and mobile phases.
Paper Chromatography
It is a type of partition chromatography. Here the fixed phase is a liquid (say H2O) adsorbed on paper. The adsorbed water acts as an immiscible liquid towards the mobile phase, which passes over the paper. The mobile phase is normally an organic liquid. There are three typical methods of performing paper chromatography namely (i) ascending (ii) descending (iii) radial/circular. Just the ascending type will be discussed.
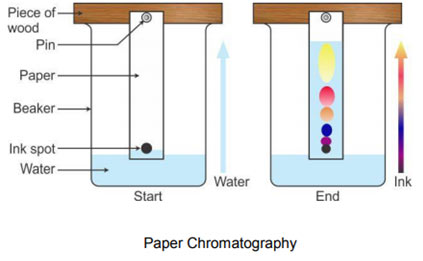
In this method, the solvent is in a pool at the bottom of a vessel in which the paper is supported and the solvent travels upwards by capillary action.
A solvent mixture especially made up in accordance with the sample to be separated, is poured into the chromatographic tank. Cover the tank to homogenize its inner environment. Take about 20 cm strip of Whatmann’s chromatographic paper No. 1 and draw on it a thin pencil line about 2.5 cm from one end. Spot a point, on the pencil line, with the sample mixture solution. To facilitate identification of the components of the mixture, spots of the known compounds may also be placed alongside.
When the areas have dried, suspend the paper with clips so that the impregnated end dips into the solvent mixture to a depth of 5-6 mm. Cover the tank. As the solvent front passes the spots, the solutes start to move upward. The rate at which they move depends on their circulation coefficients. When the solvent front has actually risen to about 3/4th of the length of the paper, remove the strip, mark the solvent front with a pencil and allow the strip to dry.
Once the paper is dried, the pattern on the paper is called a chromatogram.
The different components of the mix, if coloured, can aesthetically be identified. If colourless, the chromatogram needs to be established by chemical techniques or physical strategies used to determine the spots. Each component has a specific retardation factor called Rf value. The Rf value is connected to its circulation coefficient and is given by:
Rf= Distance travelled by a component from the original spot / Distance travelled by solvent from the original spot
The Rf values for B and C are given by:
Rf(B)= x/y
Rf (C)= z/y
Uses of Chromatography
The methods of chromatography are really useful in natural synthesis for separation, seclusion and purification of the products. They are similarly crucial in qualitative and quantitative analyses and for determination of the purity of a substance.
MCQs
- What is chromatography?
- A. The separation of colors
- B. The separation of mixtures in a laboratory
- C. A technique for cooking
- D. A method for water purification
- Answer: B
- What are the two phases in chromatography?
- A. Solid and gas
- B. Liquid and solid
- C. Mobile and stationary
- D. Adsorption and partition
- Answer: C
- What does the term “Khromatos” mean?
- A. Color separation
- B. Color writing
- C. Mobile phase
- D. Stationary phase
- Answer: B
- What is the role of the stationary phase in chromatography?
- A. It moves over the mobile phase
- B. It supports the mobile phase
- C. It is fixed and does not move
- D. It determines the solute concentration
- Answer: C
- What is the circulation coefficient (K) in chromatography?
- A. The concentration of a component in the moving phase
- B. The concentration of a component in the stationary phase
- C. The ratio of concentrations between moving and stationary phases
- D. The speed of the mobile phase
- Answer: C
- In which type of chromatography does the stationary phase consist of a liquid?
- A. Adsorption chromatography
- B. Partition chromatography
- C. Gas chromatography
- D. Liquid chromatography
- Answer: B
- What is paper chromatography?
- A. Adsorption chromatography
- B. Gas chromatography
- C. Partition chromatography
- D. Liquid chromatography on paper
- Answer: C
- How many methods of performing paper chromatography are mentioned?
- A. One
- B. Two
- C. Three
- D. Four
- Answer: C
- What is the ascending type of paper chromatography?
- A. Solvent moves downward
- B. Solvent moves upward
- C. Solvent moves in a circular pattern
- D. Solvent remains stationary
- Answer: B
- What is the role of the solvent in paper chromatography?
- A. It supports the paper
- B. It dissolves the stationary phase
- C. It carries the solute upward
- D. It determines the color of the chromatogram
- Answer: C
- What is a chromatogram?
- A. A type of paper used in chromatography
- B. A pattern formed on the paper after chromatography
- C. A tool used to measure solute concentrations
- D. A chemical used in the separation process
- Answer: B
- How is the Rf value calculated?
- A. Distance traveled by solvent / Distance traveled by a component
- B. Distance traveled by a component / Distance traveled by solvent
- C. Concentration of a component in moving phase / Concentration of that element in the fixed phase
- D. Ratio of concentrations between moving and stationary phases
- Answer: B
- What does the Rf value represent?
- A. Speed of the solvent
- B. Circulation coefficient of a component
- C. Thickness of the chromatographic paper
- D. Purity of the solvent
- Answer: B
- What is the purpose of chromatography in natural synthesis?
- A. Water purification
- B. Separation, isolation, and purification of products
- C. Cooking techniques
- D. Color writing
- Answer: B
- What role does chromatography play in qualitative and quantitative analyses?
- A. It determines the color of a substance
- B. It measures the thickness of a sample
- C. It is not useful in analyses
- D. It is crucial for qualitative and quantitative analyses
- Answer: D
- What is the significance of the Rf value in chromatography?
- A. It indicates the speed of the solvent
- B. It represents the thickness of the stationary phase
- C. It helps identify the components of the mixture
- D. It determines the purity of the solvent
- Answer: C
- Why is chromatography helpful in the analysis of food, drugs, and water samples?
- A. It determines the color of the samples
- B. It provides accurate and error-free analysis
- C. It measures the thickness of the substances
- D. It dissolves the components of the samples
- Answer: B
Summary
In conclusion, chromatography is a laboratory technique used for the separation of mixtures dissolved in fluid solvents. It involves two phases – a fixed stationary phase and a mobile phase that moves over it, which can be gas or liquid. Chromatography can be classified into adsorption chromatography with a solid stationary phase and partition chromatography with a liquid stationary phase.
The technique utilizes the distribution of solutes between these phases, governed by the circulation coefficient (K).
Paper Chromatography: Paper chromatography, a type of partition chromatography, involves a liquid fixed phase on paper and is performed in ascending, descending, or radial/circular methods. The process includes the movement of solutes as the solvent travels upwards, resulting in a chromatogram. The chromatogram helps visually identify components based on their retardation factor (Rf) values.
Chromatography finds extensive applications in natural synthesis, separation, isolation, and purification of products, as well as in qualitative and quantitative analyses for determining substance purity.