Carbohydrates are found abundantly in mostly all living creatures. Carbohydrates are commonly distributed both in plants and animals.
The cellulose of wood, paper, and cotton, starches within cereals, root tubers, cane sugar, and sugar in milk are typical examples of carbohydrates.
Carbohydrates play structural and functional roles. Carbohydrates are the principal source of energy. Some carbohydrates are the chief elements of cell walls in plants and microorganisms such as chitin in fungi. Chemically they are carbon dioxide, oxygen, and hydrogen.
The empirical formula of most simple carbohydrates is [CH2O] n. Usually, hydrogen-oxygen atoms are in ratio of 2:1 (like in water) hence the name “carbohydrate”, i.e. hydrated carbons.
They are also called “saccharides”. In Greek, saccharon means sugar.
Although many common carbohydrates confirm the empirical formula [CH2O] n, others like deoxyribose, rhamnohexos do not. Some carbohydrates also contain nitrogen, phosphorus, or sulphur.
- 1) Definition
- 2) Structure
- 3) Classification
- 4) Monosaccharides (Greek: Mono = one)
- 5) Glucose
- 6) Structure of glucose
- 7) Oligosaccharides (Greek: oligo = few)
- 8) Disaccharides
- 9) Maltose
- 10) Sucrose (Common Table Sugar)
- 11) POLYSACCHARIDES (GLYCANS)
- 12) Homopolysaccharides or Homoglycans
- 13) Heteropolysaccharides or Heteroglycans
- 14) Sources of carbohydrates
- 15) Functions of carbohydrates
- 16) FAQs on Carbohydrates
- 17) You may also like to learn:
Definition
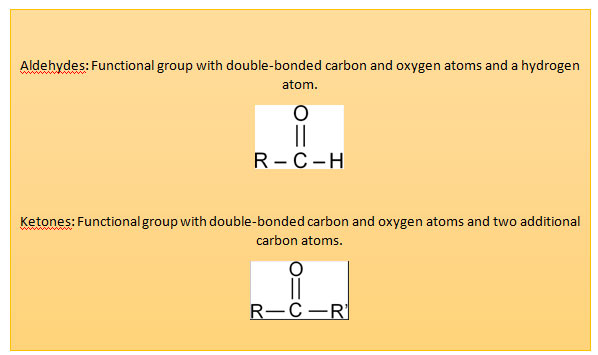
Carbohydrates may be defined chemically as an aldehyde or ketone derivatives of polyhydroxy (more than one hydroxy group) alcohol or as compounds that yield these derivatives on hydrolysis.
Structure
Carbohydrates may be aldehydes or ketones
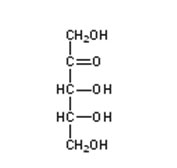
Aldehyde carbohydrates
Glyceraldehyde
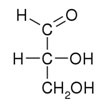
Ribose
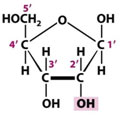
Glucose
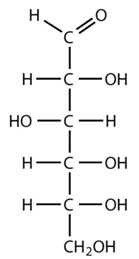
Ketonic carbohydrates
Dihydroxyacetone
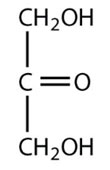
Ribulose
Fructose
Classification
Carbohydrates are classified into three groups:
- Monosaccharides
- Oligosaccharides
- Polysaccharides
Monosaccharides (Greek: Mono = one)
All these are simple sugars. They are sweet in nature and therefore are easily soluble in water. They also cannot be hydrolyzed into sugars. Chemically they’re either polyhydroxy aldehydes or ketones. All carbon atoms in a monosaccharide except one, possess a hydroxyl group. The rest of the carbon molecule is a part of an aldehyde group or a keto group. The glucose with aldehyde functional group is called aldo-sugar; as well as the keto functional group as keto-sugar. They are subdivided into two classes as follows:
- Depending upon the number of carbon atoms they
possess, e.g.
– Trioses– Tetroses – Pentoses– Hexoses– Heptoses.
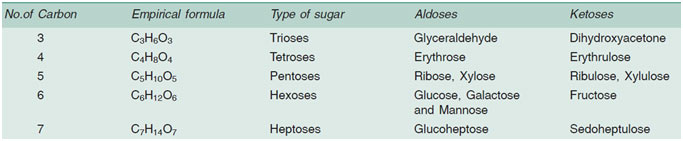
- Depending upon the functional aldehyde (CHO) or
ketone (C=O) group present:
- – Aldoses
- – Ketoses
(as mentioned above in the subheading “structure”)
Trioses are intermediate in respiration and photosynthesis. Tetroses are rare in nature and mostly found in some bacteria. Pentoses and hexoses are the most common. From the biological point of view the most important hexose is glucose. It is an aldose sugar.
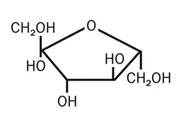
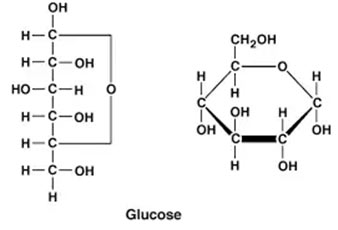
Glucose
Physiologically and biomedically, glucose is the most essential monosaccharide. In free state, sugar is found in every fruit, being abundant in dates, seeds, figs, and grapes.
Our blood glucose generally comprises 0.08% sugar levels. In combined form, it is found in many disaccharides and polysaccharides. Starch, cellulose, and glycogen yield glucose on complete hydrolysis.
Glucose is naturally produced in green plants which take carbon dioxide from the air and water from the soil to synthesize glucose by the process of photosynthesis.
The energy is stored in the glucose molecules as chemical energy and becomes available in all organisms when it is oxidized in the body.
Structure of glucose
Oligosaccharides (Greek: oligo = few)
Oligosaccharides consist of a short chain of monosaccharide units (2 to 10 units), joined together by a characteristic bond called glycosidic bond which, on hydrolysis, gives two to ten molecules of simple sugar (monosaccharide) units. Oligosaccharides are subdivided into different groups based on the number of monosaccharide units present.
The disaccharides which have two monosaccharide units are the most abundant in nature. Oligosaccharides with more than three subunits are usually found in glycoproteins; such as blood group antigens.
Disaccharides
- Disaccharides consist of two monosaccharide units.
- They are crystalline, water soluble, and sweet to taste.
- They are subclassified on the basis of the presence or absence of free reducing (aldehyde or ketone) groups
- Reducing disaccharides with free aldehyde or keto group, e.g. maltose, lactose
- Non-reducing disaccharides with no free aldehyde or keto group, e.g. sucrose.
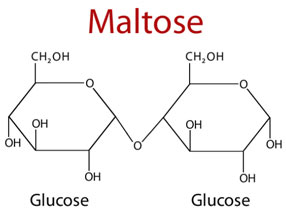
Maltose
- Maltose contains two glucose molecules, joined by the glycosidic linkage between C-1 (the anomeric carbon) of one glucose residue and C-4 of the other, leaving one free anomeric carbon of the second glucose residue, which can act as a reducing agent. Thus, maltose is a reducing disaccharide.
- The numerical description like (1 → 4) of the glycosidic bond represents the number of carbon atoms that connect the two sugars.
- Maltose is produced as an intermediate product in the digestion of starch and glycogen by the action of the enzyme α-amylase.
| Anomeric carbon
The carbon derived from carbonyl compound as an aldehyde or ketone functional group is called anomeric carbon. |
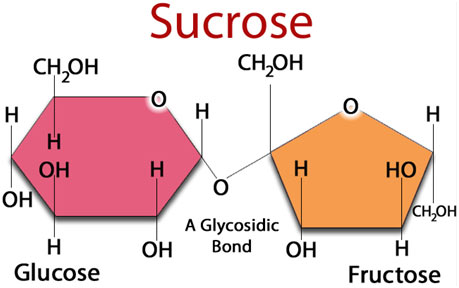
Sucrose (Common Table Sugar)
- Sucrose is a disaccharide of glucose and fructose. It is formed by plants but not by human beings. Sucrose is an intermediate product of photosynthesis. It is the commonly used table sugar.
- The anomeric carbon of both glucose and fructose are involved in the glycosidic bond. Sucrose is, therefore, a non-reducing sugar.
- Sucrose is hydrolyzed to fructose and glucose by an enzyme sucrase which is also called invertase.
POLYSACCHARIDES (GLYCANS)
Carbohydrates composed of ten or more monosaccharide units or their derivatives (such as amino sugars and uronic acids) are generally classified as polysaccharides. Polysaccharides are colloidal in size. In polysaccharides, monosaccharide units are joined together by glycosidic linkages. Another term for polysaccharides is “glycans”.
Polysaccharides are subclassified into two groups:
- Homopolysaccharides (Homoglycans): When a polysaccharide is made up of several units of one and the same type of monosaccharide unit only, it is called homopolysaccharide.
- Heteropolysaccharides (Heteroglycans): They contain two or more different types of monosaccharide units or their derivatives.
Homopolysaccharides or Homoglycans
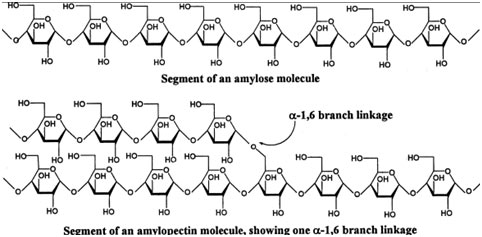
Starch
It is the storage form of glucose in plants, e.g. in potato, in grains and seeds, and in many fruits. Starch is composed of two constituents viz. amylose and amylopectin.
Amylose
Amylose is a linear polymer of D-glucose units joined by α-1 → 4 glycosidic linkages.
Amylopectin
Amylopectin is structurally identical to those of amylose (α-1→ 4 glycosidic linkages) but with side chains joining them by α-1→ 6 linkages. Thus, amylopectin is a branched polymer having both α-(1 → 4) and α-(1 → 6) linkages. The branch points in amylopectin are created by α-1 → 6 bonds and occur at an interval of 20 to 30 units of glucose.
Heteropolysaccharides or Heteroglycans
Glycosaminoglycans (GAGs) or
Mucopolysaccharides
A GAG is an unbranched heteropolysaccharide, made up of repeating disaccharides. GAG is a polymer of [uronic acid-amino sugar] n.
This polymer is attached covalently to extracellular proteins called core protein to form proteoglycans. One component of which is always an amino sugar (hence the name glycosaminoglycans).
The other component of the repeating disaccharide (except in the case of keratan sulfate) is uronic acid, either L-glucuronic acid or its epimer L-iduronic acid.
They are major components of the extracellular matrix or ground substance. They act as “molecular sieves”, determining which substances enter and leave cells.
Sources of carbohydrates
Carbohydrates are available in a broad range of nutritious, healthy, and noxious foods including bread, legumes, milk, popcorn, potatoes, cookies, soft beverages, corn, and cherry pie.
They also come in many different forms. The most typical and abundant kinds are sugars, fibers, and starches.
Naturally, they can be obtained from sugarcane, vegetables like potatoes, grains, wheat, oats, barley, rice, and fruits. Dates are enriched with carbohydrates(glucose).

Functions of carbohydrates
- Foods high in carbohydrates are essential for healthy diet. Carbohydrates provide the body with glucose, which is converted to energy (ATP) used to support body functions, metabolism, and physical activity.
- The storage form of energy, e.g. glycogen in animal tissue and starch in plants
- Serve as a structural component, e.g. glycosaminoglycans humans, cellulose in plants, and chitin in insects
- Non-digestible carbohydrates like cellulose, serve as dietary fibers
- A constituent of nucleic acids RNA and DNA, e.g. ribose and deoxyribose sugar
- Play a role in lubrication, cellular intercommunication, and immunity
- Carbohydrates are also involved in detoxification, e.g. glucuronic acid.
FAQs on Carbohydrates
1. What are carbohydrates and where are they found?
- Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen. They are abundantly found in both plants and animals. Examples include cellulose in plants, starches in cereals, cane sugar, and sugar in milk.
2. What is the chemical composition of carbohydrates?
- Carbohydrates are composed of carbon, hydrogen, and oxygen. The empirical formula of most simple carbohydrates is [CH2O]n, reflecting the 2:1 ratio of hydrogen to oxygen, similar to water.
3. Why are carbohydrates called “hydrated carbons” or “saccharides”?
- Carbohydrates are called “hydrated carbons” because they have a 2:1 ratio of hydrogen to oxygen, resembling water. The term “saccharides” is derived from the Greek word “saccharon,” meaning sugar.
4. How are carbohydrates classified based on structure?
- Carbohydrates can be classified into aldehyde carbohydrates (aldoses) and ketonic carbohydrates (ketoses). Examples of aldehyde carbohydrates include glyceraldehyde and ribose, while dihydroxyacetone and fructose are examples of ketonic carbohydrates.
5. What are the three main groups of carbohydrates?
- Carbohydrates are classified into monosaccharides (simple sugars), oligosaccharides (short chains of monosaccharide units), and polysaccharides (long chains of monosaccharide units or their derivatives).
6. What is the significance of glucose in biology?
- Glucose is a physiologically and biomedically essential monosaccharide. It is a primary source of energy, found in fruits, and plays a crucial role in processes like photosynthesis. Glucose is also a building block for more complex carbohydrates.
7. How are disaccharides classified, and what are some examples?
- Disaccharides are classified based on the presence or absence of free reducing groups. Examples of reducing disaccharides with free aldehyde or keto groups include maltose and lactose, while sucrose is a non-reducing disaccharide.
8. What are homopolysaccharides and heteropolysaccharides?
- Homopolysaccharides consist of repeated units of the same type of monosaccharide, such as starch with amylose and amylopectin. Heteropolysaccharides contain two or more different types of monosaccharide units or their derivatives.
9. What are the functions of carbohydrates in the body?
- Carbohydrates serve as a source of energy, a storage form of energy (glycogen in animals, starch in plants), structural components (e.g., cellulose, glycosaminoglycans), and play roles in lubrication, cellular communication, immunity, and detoxification.
10. What are common sources of carbohydrates in the diet?
- Carbohydrates are found in various foods such as bread, legumes, milk, potatoes, cookies, fruits, and more. They come in different forms, including sugars, fibers, and starches, and are crucial for a healthy diet.
Summary of Carbohydrates
Carbohydrates are essential complex sugar molecules present in all living things. Chemically they are aldehyde or ketone derivatives of polyhydroxy (more than one hydroxy group) alcohols or as compounds that yield these derivatives on hydrolysis with the empirical formula for most simple carbohydrates is [CH2O] n.
Carbohydrates are classified into three groups i.e., monosaccharides, oligosaccharides, and polysaccharides. Monosaccharides are simple sugars and cannot be hydrolyzed. Oligosaccharides consist of a short chain of monosaccharide units (2 to 10 units), joined together by a characteristic bond called glycosidic bond. Polysaccharides composed of ten or more monosaccharide units or their derivatives such as amino sugars and uronic acids. Glucose, fructose, maltose, sucrose, starch, glycogen, etc., are all important carbohydrates.
They are present in almost all living organisms either plants or animals. Carbohydrates are of very important nutritional value. They have structural and functional roles. Serve as storage compounds. Are involved in detoxification. They are constituents of RNA and DNA.
