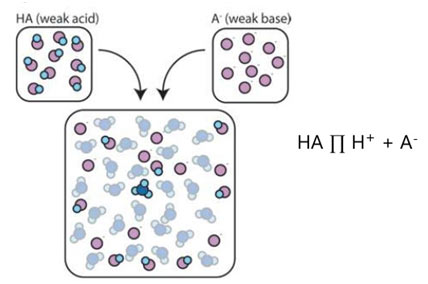
A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in pH. To put it simply, a buffer is an aqueous solution of either a weak acid and its conjugate base or a weak base and its conjugate acid.A buffer might also be called a pH buffer, hydrogen ion buffer, or buffer solution. The Henderson-Hasselbalch equation may be used to gauge the approximate pH of a buffer.
To use the equation, the preliminary concentration or stoichiometric concentration is gotten in instead of the equilibrium concentration.
The basic form of a buffer chemical reaction is:
HA ⇌ H+ + A −
Types of Buffer Solution
Acidic buffer
Acid buffer solutions have a pH of less than 7. It is generally made from a weak acid and among its salts (frequently called conjugate *). Typically utilized acidic buffer solutions are a mix of ethanoic acid and salt ethanoate in solution, which have a pH of 4.76 when mixed in equal molar concentrations. You can change the pH of the buffer solution by altering the ratio of acid to salt, or by picking a different acid and among its salts.
Alkaline buffer
Alkaline buffer solutions have a pH greater than 7 and are made from a weak base and one of its salts. Avery commonly used the example of an alkaline buffer solution is a mixture of ammonia and ammonium chloride solution. If these were mixed in equivalent molar proportions, the solution would have a pH of 9.25.
Working of Buffer Solution
Let’s take the example of a solution of acetic acid (CH3COOH) and salt acetate (CH3COONa). Here, acetic acid is weakly ionized while sodium acetate is almost completely ionized. The formulas are offered as follows:
CH3COOH H+ + CH3COO–
CH3COONa Na+ + CH3COC–
To this, if you add a drop of a strong acid like HCl, the H+ ions from HCl combine with CH3COO– to offer feebly ionized CH3COOH. Thus, there is a really small modification in the pH value. Now, if you add a drop of NaOH, the OH– ions react with the complimentary acid to provide undissociated water particles.
CH3COOH + OH– CH3COO– + H2O
In this way, the OH– ions of NaOH are eliminated and the pH is practically unchanged.
Preparing a Buffer Solution
There are a couple of ways to prepare a buffer solution of a specific pH. In the very first approach, prepare a solution with acid and its conjugate base by dissolving the acid kind of the buffer in about 60% of the volume of water required to get the final solution volume. Then, measure the pH of the solution utilizing a pH probe. The pH can be changed approximately to the preferred value using a strong base like NaOH.
If the buffer is made with a base and its conjugate acid, the pH can be changed using a strong acid like HCl. As soon as the pH is appropriate, water down the solution to the last preferred volume.
Alternatively, you can prepare solutions of both the acid form and base form of the solution. Both solutions must include the same buffer concentration as the concentration of the buffer in the final solution. To get the final buffer, add one solution to the other while monitoring the pH.
Significance of Buffer Solution
- Buffer solutions are utilized for comparing calorimetrically the hydrogen ion concentration of unknown solutions.
- Acetic acid-sodium acetate is used in the removal of phosphate radical throughout the qualitative analysis of the solution.
- Buffer is utilized for the precipitation of hydroxides of the third group of qualitative analysis.
- Bicarbonate Buffer
The maintenance of blood pH is controlled using the bicarbonate buffer. This system includes carbonic acid and bicarbonate ions. When the blood pH drops into the acidic range, this buffer acts to form carbon dioxide gas. The lungs expel this gas out of the body during the procedure of respiration. During alkaline conditions, this buffer brings pH back to neutral by triggering the excretion of the bicarbonate ions through the urine.
- The respiratory pigment present in blood, hemoglobin, likewise has buffering action within tissues. It can bind with either protons or oxygen at a given point in time. Binding of one release the other. At the time of a workout or exercise, protons are generated in excess. Hemoglobin helps in the buffering action by binding these protons, and at the same time releasing molecular oxygen.
- In industries, buffer solutions are used in alcoholic fermentation (pH 5 to 6.5), tanning of leather, electroplating, manufacture of sugar, paper manufacturing, and so on.
Multiple Choice Questions (MCQs) with Answers
Definition of Buffer Solution:
- What is a buffer solution?
- A. Pure water
- B. A solution resistant to changes in pH
- C. A strong acid solution
- D. An alkaline solution
- Answer: B
- Which equation can be used to estimate the pH of a buffer solution?
- A. Avogadro’s equation
- B. Henderson-Hasselbalch equation
- C. Boyle’s law
- D. Charles’s law
- Answer: B
Types of Buffer Solution:
- What is the pH range of acidic buffer solutions?
- A. Greater than 7
- B. Exactly 7
- C. Less than 7
- D. Equal to 14
- Answer: C
- Which example represents an alkaline buffer solution?
- A. Hydrochloric acid
- B. Ammonia and ammonium chloride
- C. Sodium hydroxide
- D. Sulfuric acid
- Answer: B
Working of Buffer Solution:
- In a buffer solution of acetic acid and sodium acetate, what happens when a drop of HCl is added?
- A. pH remains unchanged
- B. pH increases significantly
- C. pH decreases significantly
- D. Formation of gas bubbles
- Answer: A
- How does the buffer solution resist changes in pH when NaOH is added?
- A. OH– ions react with the free acid
- B. H+ ions react with the conjugate base
- C. Both A and B
- D. Formation of a precipitate
- Answer: C
Preparing a Buffer Solution:
- What is the first step in preparing a buffer solution using the first method mentioned?
- A. Dissolving the base form of the buffer
- B. Dissolving the acid form of the buffer
- C. Measuring the pH of the solution
- D. Adding NaOH directly to water
- Answer: B
- How is the pH adjusted in the preparation of a buffer solution with a base and its conjugate acid?
- A. Using a strong acid like HCl
- B. Using a strong base like NaOH
- C. By diluting the solution
- D. Adding a catalyst
- Answer: A
Significance of Buffer Solution:
- What is the significance of acetic acid-sodium acetate buffer in qualitative analysis?
- A. Removal of phosphate radical
- B. Precipitation of hydroxides
- C. Blood pH regulation
- D. Calorimetric comparison
- Answer: A
- How does the bicarbonate buffer system maintain blood pH during acidic conditions?
- A. Expelling carbon dioxide through respiration
- B. Formation of oxygen gas
- C. Precipitation of bicarbonate ions
- D. Excretion through urine
- Answer: A
Industries and Miscellaneous:
- In which industry are buffer solutions used for alcoholic fermentation?
- A. Textile industry
- B. Food industry
- C. Leather tanning industry
- D. Pharmaceutical industry
- Answer: B
- What role does hemoglobin play in buffering during exercise?
- A. Binding protons and releasing carbon dioxide
- B. Binding oxygen and releasing carbon dioxide
- C. Binding and releasing water molecules
- D. Precipitating bicarbonate ions
- Answer: B
- Which is NOT a type of buffer mentioned in the tutorial?
- A. Acidic buffer
- B. Alkaline buffer
- C. Neutral buffer
- D. Bicarbonate buffer
- Answer: C
Problem/Solutions for “Buffer Solutions”
Problem 1: Insufficient Buffer Capacity
Problem: The buffer solution’s capacity to resist changes in pH is inadequate for certain applications, leading to undesired pH variations.
Solution: Increase the concentration of the weak acid or base and its conjugate salt in the buffer solution. This enhances the buffering capacity, making the solution more effective in maintaining pH stability.
Problem 2: pH Drift Over Time
Problem: The buffer solution experiences gradual pH drift over an extended period, compromising its reliability in maintaining a constant pH.
Solution: Periodically monitor and readjust the pH of the buffer solution by adding small amounts of strong acid or base as needed. This ensures the continued effectiveness of the buffer over time.
Problem 3: Incorrect pH Range
Problem: The buffer solution’s pH does not fall within the desired range for a specific application, leading to inefficiency.
Solution: Choose a different weak acid-base pair with a pKa value that aligns with the desired pH range. Additionally, adjust the ratio of acid to salt or base to salt in the buffer to fine-tune the pH.
Problem 4: Limited Shelf Life
Problem: Buffer solutions may degrade or lose their effectiveness over time, especially during storage.
Solution: Store buffer solutions in appropriate conditions, such as refrigeration or a controlled environment, to extend their shelf life. Regularly check the solution’s pH and remix or replace as needed.
Problem 5: Compatibility Issues in Biological Systems
Problem: Buffer solutions used in biological applications may interfere with biochemical reactions or cellular processes.
Solution: Select buffer components that are biologically compatible and do not interfere with the specific biological system. Consider factors like temperature, ionic strength, and the nature of the biological molecules involved.
Summary
Definition of Buffer Solution: A buffer solution is an aqueous solution containing a weak acid and its salt or a weak base and its salt, resistant to changes in pH. Also known as a pH buffer or hydrogen ion buffer, buffers can be evaluated using the Henderson-Hasselbalch equation. The general buffer chemical reaction is expressed as: .
Types of Buffer Solution:
- Acidic Buffer: Has a pH less than 7, often made from a weak acid and its salt. Example: Ethanoic acid and sodium ethanoate.
- Alkaline Buffer: Has a pH greater than 7, made from a weak base and its salt. Example: Ammonia and ammonium chloride.
Working of Buffer Solution: Using the example of acetic acid (CH₃COOH) and sodium acetate (CH₃COONa), when a strong acid (HCl) is added, the pH changes minimally. Adding a strong base (NaOH) removes OH⁻ ions, keeping the pH nearly unchanged.
Preparing a Buffer Solution: Preparation methods involve dissolving the acid form or base form in water, adjusting pH with strong base or acid, and diluting to the desired volume. Alternatively, solutions of acid and base forms are mixed, maintaining the desired concentration.
Significance of Buffer Solution:
- Used for comparing hydrogen ion concentration calorimetrically.
- Acetic acid-sodium acetate aids in phosphate radical removal in qualitative analysis.
- Buffer assists in the precipitation of hydroxides in qualitative analysis.
- Bicarbonate buffer system regulates blood pH, expelling carbon dioxide during acidic conditions and triggering bicarbonate ion excretion during alkaline conditions.
- Hemoglobin in blood provides buffering during exercise.
- In industries, buffer solutions are used in processes such as alcoholic fermentation, leather tanning, electroplating, sugar manufacturing, and paper production.
Buffers play a crucial role in maintaining stable pH conditions in various applications, showcasing their significance in both biological and industrial processes.